
Hydrogen Storage Physisorption <o.1eV,vdW force Phys-chemisorption transition state Defect and Doping Chemisorption 2-3 eV,chemical bond 0.2-0.4eV Graphene-H2,Physisorption >Graphene/Nanotubes >Structural efects E,≈60meV/80meV Vacancy 2000 20%T >Inside C60/(5,5)tubes 5-7 defect Eb≈0.2eV 50 >Substitutional doping Boron-doped graphene 0 E,≈80meV -50 -100 3 Graphane,Chemisorption Binding Distance [A]
Physisorption < 0.1 eV, vdW force Phys-chemisorption transition state 0.2-0.4 eV Chemisorption 2-3 eV, chemical bond Graphene-H2 , Physisorption Graphane, Chemisorption Hydrogen Storage Defect and Doping
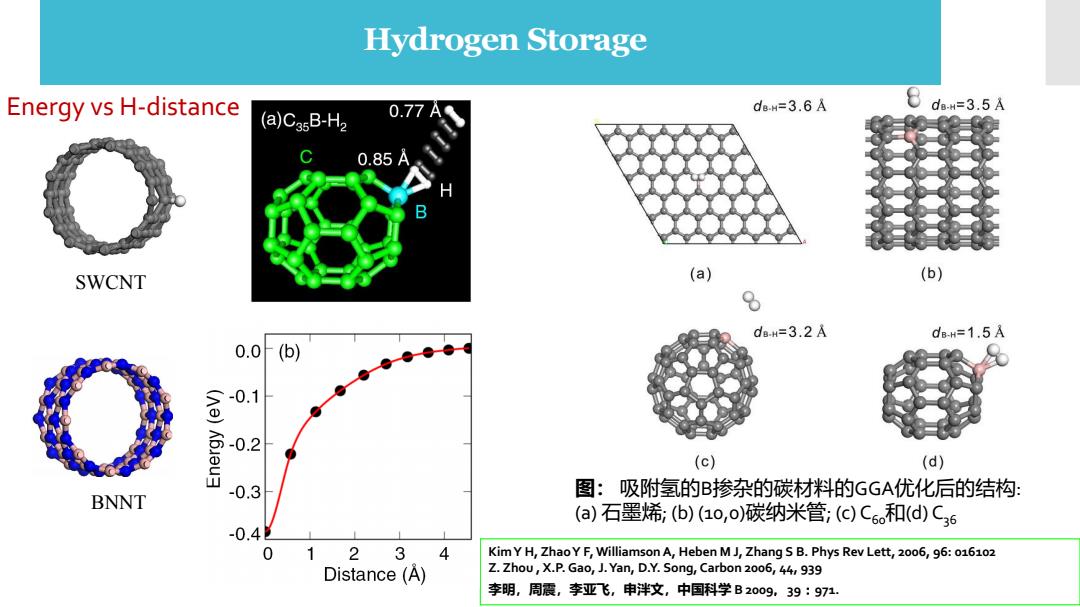
Hydrogen Storage Energy vs H-distance (a)CagB-H2 0.77 de-H=3.6A 8dw=3.5A 0.85A SWCNT (a) (b) 6 d8H=3.2A dB-H=1.5A 0.0 (b) -0.1 -0.2 (c) (d) 图:吸附氢的B掺杂的碳材料的GGA优化后的结构: BNNT -0.3 (a)石墨烯,(b)(1o,o)碳纳米管;()Co和(d)C6 -0.4 0 12 3 4 Kim Y H,ZhaoY F,Williamson A,Heben MJ,Zhang S B.Phys Rev Lett,2006,96:016102 Distance(A) Z.Zhou,X.P.Gao,J.Yan,D.Y.Song,Carbon 2006,44,939 李明,周震,李亚飞,申泮文,中国科学B2009,39:971
B-doped carbon nanostructures 图: 吸附氢的B掺杂的碳材料的GGA优化后的结构: (a) 石墨烯; (b) (10,0)碳纳米管; (c) C60和(d) C36 Kim Y H, Zhao Y F, Williamson A, Heben M J, Zhang S B. Phys Rev Lett, 2006, 96: 016102 Z. Zhou , X.P. Gao, J. Yan, D.Y. Song, Carbon 2006, 44, 939 李明,周震,李亚飞,申泮文,中国科学 B 2009,39:971. Hydrogen Storage SWCNT BNNT Energy vs H-distance
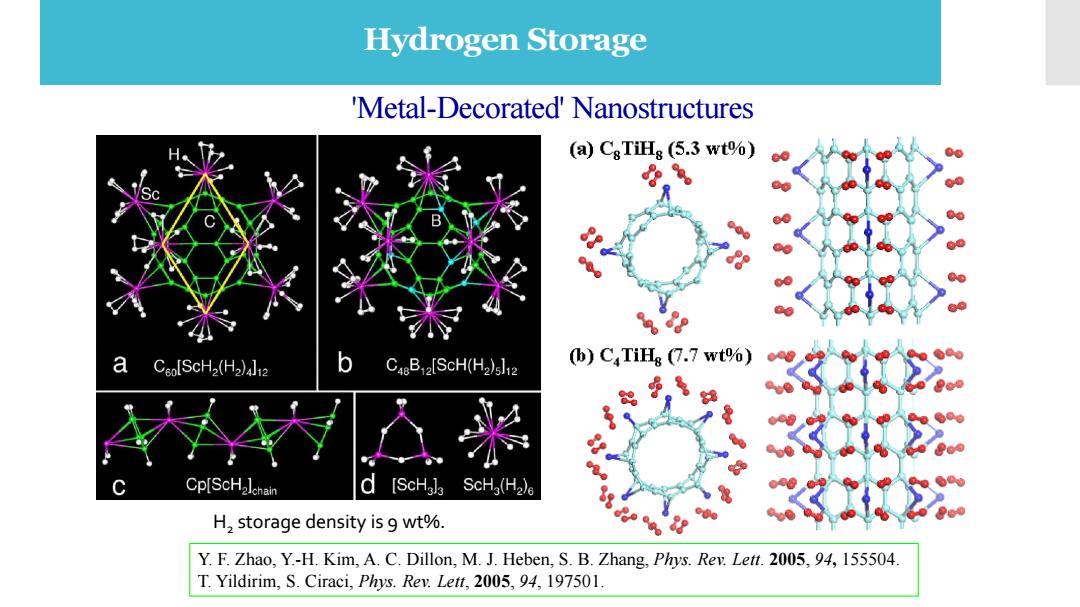
Hydrogen Storage 'Metal-Decorated'Nanostructures (a)CsTiHg (5.3 wt%) a Cao[SCH2(H2)12 b Ca8B2[SCH(H2)s]12 (b)CTiHg (7.7 wt%) Cp[SCH2lchain d [ScHala ScHa(H2)a H,storage density is 9 wt%. Y.F.Zhao,Y.-H.Kim,A.C.Dillon,M.J.Heben,S.B.Zhang,Phys.Rev.Lett.2005,94,155504 T.Yildirim,S.Ciraci,Phys.Rev.Lett,2005,94,197501
'Metal-Decorated' Nanostructures H2 storage density is 9 wt%. Y. F. Zhao, Y.-H. Kim, A. C. Dillon, M. J. Heben, S. B. Zhang, Phys. Rev. Lett. 2005, 94, 155504. T. Yildirim, S. Ciraci, Phys. Rev. Lett, 2005, 94, 197501. Hydrogen Storage
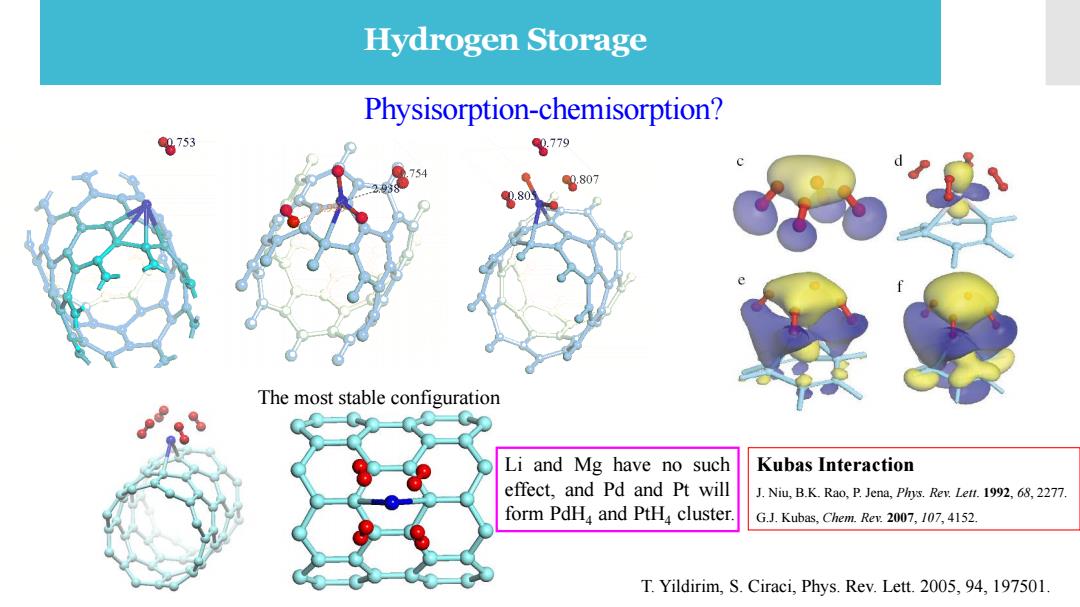
Hydrogen Storage Physisorption-chemisorption? 0753 8779 0,807 @0.80 The most stable configuration Li and Mg have no such Kubas Interaction effect,and Pd and Pt will J.Niu,B.K.Rao,P.Jena,Phys.Rev.Lett.1992,68,2277. form PdHa and PtH cluster G.J.Kubas,Chem.Rev.2007,107,4152. T.Yildirim,S.Ciraci,Phys.Rev.Lett.2005,94,197501
Physisorption-chemisorption? The most stable configuration T. Yildirim, S. Ciraci, Phys. Rev. Lett. 2005, 94, 197501. Hydrogen Storage Li and Mg have no such effect, and Pd and Pt will form PdH4 and PtH4 cluster. Kubas Interaction J. Niu, B.K. Rao, P. Jena, Phys. Rev. Lett. 1992, 68, 2277. G.J. Kubas, Chem. Rev. 2007, 107, 4152