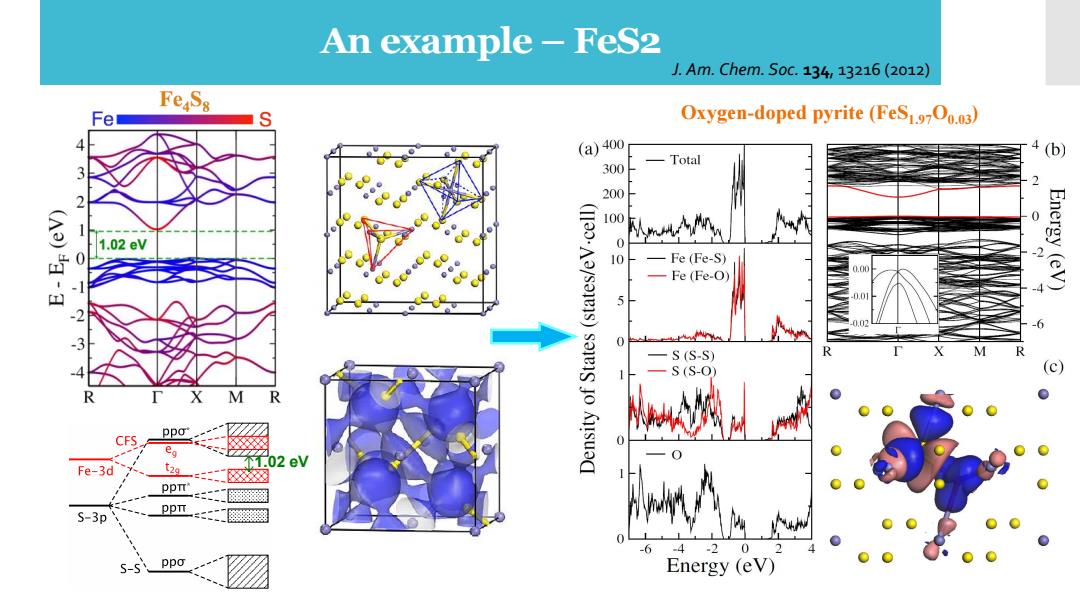
An example-FeS2 J.Am.Chem.S0c.134,13216(2o12) FeS8 Oxygen-doped pyrite(FeS1700.03) (a)400 Total (b) 300 2 200 100 0 (A)- 1.02eV 0 Fe(Fe-S) 2 0.00 Energy(eV) -Fe(Fe-O) 0.0 0.02 S(S-S) R S(S-O) M R CFS ppo ea 1.02 eV Fe-3d PpTT S-3p ppi -2 0 5-s ppo Energy (eV)
1.02 eV An example – FeS2 J. Am. Chem. Soc. 134, 13216 (2012) Oxygen-doped pyrite (FeS1.97O0.03)

An example-FeS2 J.Am.Chem.Soc.134,13216(2012 3 9 10 11 9 10 11 Atomic structures of FeS1.812500.187s alloys in a Atomic structures of FeS1.7500.2s alloys in a 2x2x2 supercell. 2x2×2 supercell
An example – FeS2 Atomic structures of FeS1.8125O0.1875 alloys in a 2×2×2 supercell. J. Am. Chem. Soc. 134, 13216 (2012) Atomic structures of FeS1.75O0.25 alloys in a 2×2×2 supercell
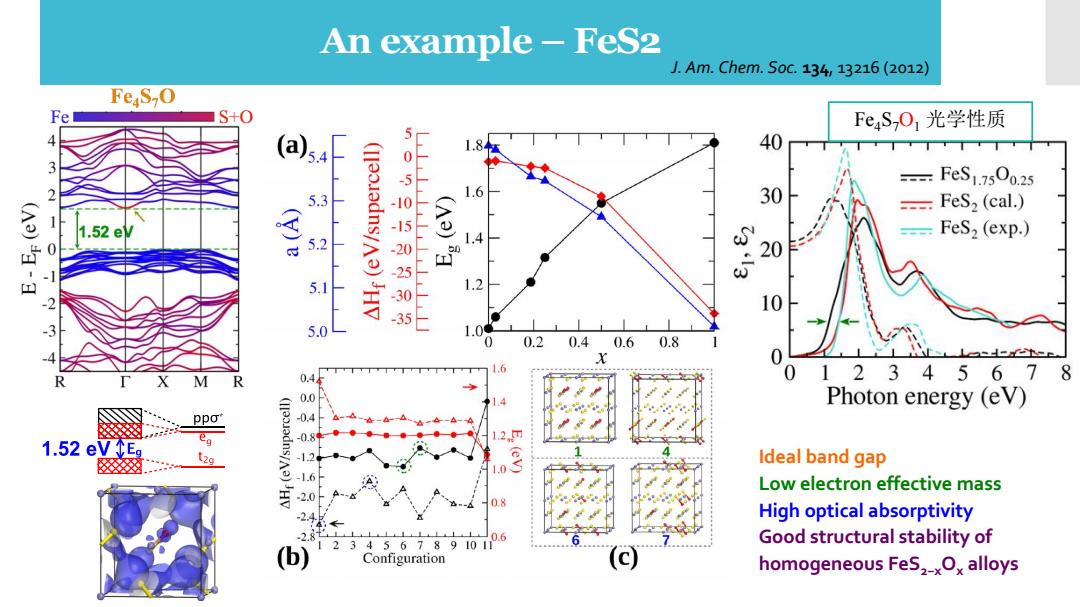
An example-FeS2 J.Am.Chem.S0c.134,13216(2012) FeS-O Fe S+0 FeSO1光学性质 (a54 40 3 (II35.adns/A3)JHV 50500520505 FeS1.750o.25 5.3 6 30 -FeS2 (cal.) 1.52e -FeS2 (exp.) 5.2 20 5.1 1.2 10 5.0 1.0 0.20.40.60.8 0 1.6 0.4 01234567 8 Photon energy (eV) ppo 1.2 1.52 eVEg t2a -12 E.(ev) Ideal band gap 1.6 04 Low electron effective mass High optical absorptivity 28 0.6 3456789101 Good structural stability of (b) Configuration homogeneous FeS2-xOx alloys
Fe4S7O1 光学性质 Ideal band gap Low electron effective mass High optical absorptivity Good structural stability of homogeneous FeS2−xOx alloys An example – FeS2 J. Am. Chem. Soc. 134, 13216 (2012)
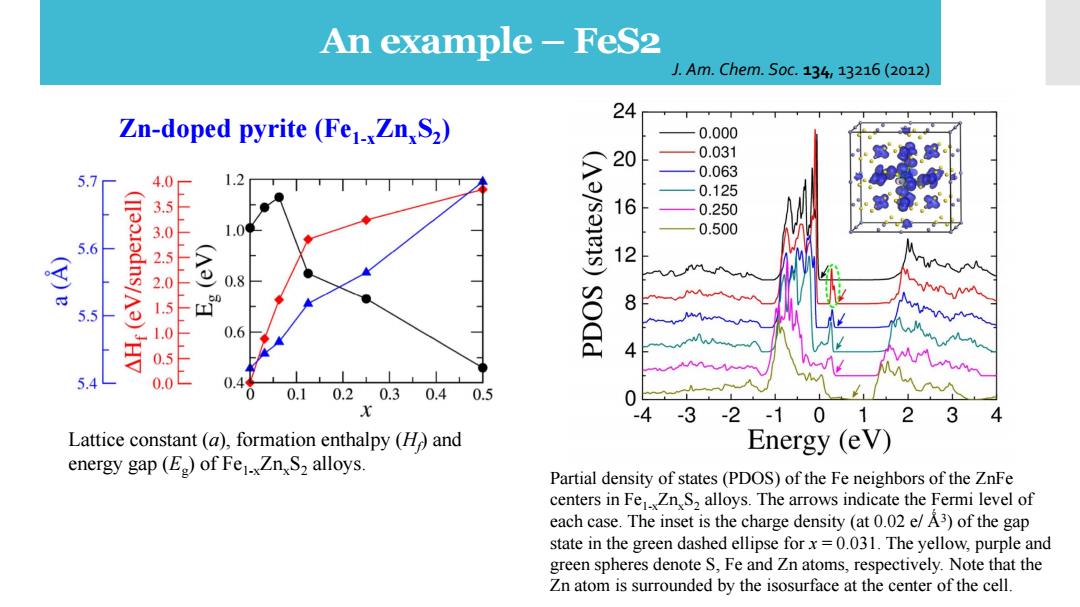
An example-FeS2 J.Am.Chem.S0c.134,13216(2012) 24 Zn-doped pyrite (Fe1xZn,S2) 0.000 0.031 5.7 4.0 20 0.063 0.125 1 0.250 0.500 5.6 12 0.8 8 5.5 500150150 0.6 sodd 0.0 0.44 0.10.20.3 0.40.5 x 3 -2-10 12 3 4 Lattice constant (a),formation enthalpy (H and Energy (eV) energy gap(E)of Fe1.Zn S2 alloys. Partial density of states(PDOS)of the Fe neighbors of the ZnFe centers in FeZnS2 alloys.The arrows indicate the Fermi level of each case.The inset is the charge density (at 0.02 e/A3)of the gap state in the green dashed ellipse forx=0.031.The yellow,purple and green spheres denote S,Fe and Zn atoms,respectively.Note that the Zn atom is surrounded by the isosurface at the center of the cell
An example – FeS2 Partial density of states (PDOS) of the Fe neighbors of the ZnFe centers in Fe1-xZnxS2 alloys. The arrows indicate the Fermi level of each case. The inset is the charge density (at 0.02 e/ Ǻ3 ) of the gap state in the green dashed ellipse for x = 0.031. The yellow, purple and green spheres denote S, Fe and Zn atoms, respectively. Note that the Zn atom is surrounded by the isosurface at the center of the cell. J. Am. Chem. Soc. 134, 13216 (2012) Lattice constant (a), formation enthalpy (Hf ) and energy gap (Eg ) of Fe1-xZnxS2 alloys. Zn-doped pyrite (Fe1-xZnxS2 )
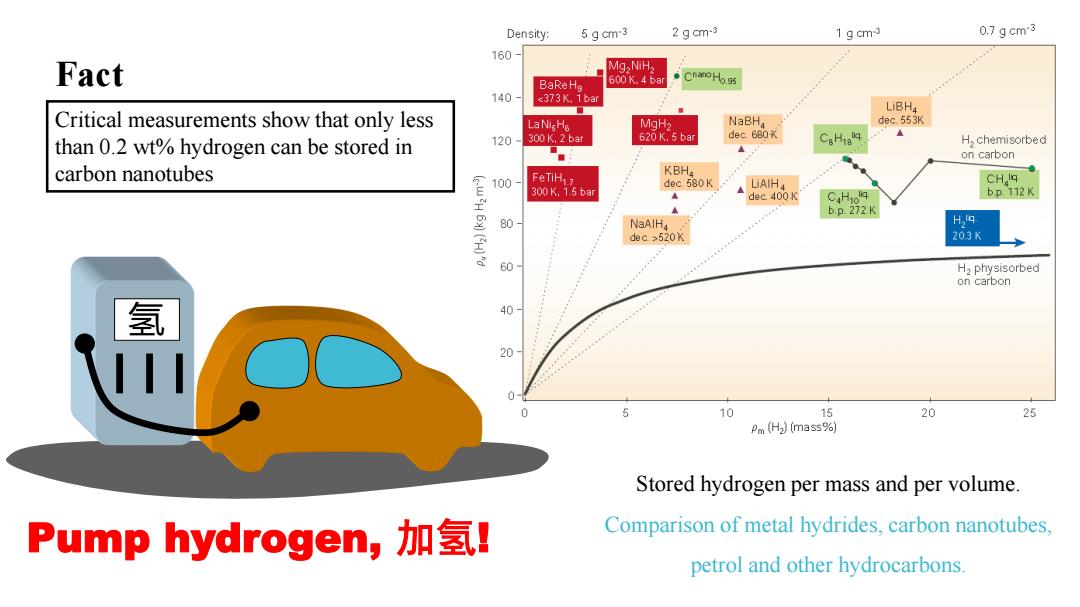
Density: 5 g cm-3 2 g cm-3 1 gcm3 0.7gcm-3 160 Fact Mg,NiH, BaRe Ho 600 K.4 bar Cnano Ho.95 140 <373K.1bar LiBH, Critical measurements show that only less MgHa NaBHa dec.553K than 0.2 wt%hydrogen can be stored in 120 300K,2 bar 620 K.5 bar dec.680K CaHie H,chemisorbed ◆◆ on carbon carbon nanotubes KBH 100 FeTiH dec 580K LAIH CHq 300K,15ba dec.400K CH10 b.p.112K 2H b.p.272K 80 NaAIHa H29 dec >520K 203K 60 H,physisorbed on carbon 氢 % 20 5 10 15 20 25 Pm (H2)(mass%) Stored hydrogen per mass and per volume. Pump hydrogen,加氢l Comparison of metal hydrides,carbon nanotubes, petrol and other hydrocarbons
Stored hydrogen per mass and per volume. Comparison of metal hydrides, carbon nanotubes, petrol and other hydrocarbons. 氢 Pump hydrogen, 加氢! Fact Critical measurements show that only less than 0.2 wt% hydrogen can be stored in carbon nanotubes