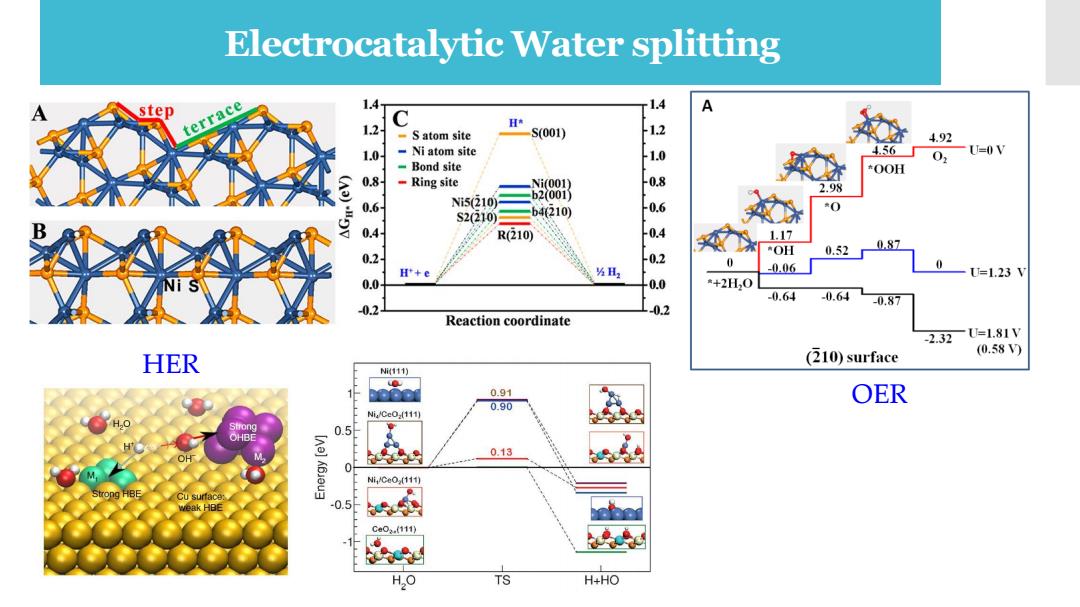
Electrocatalytic Water splitting step terrace 1.4 1.4 12 C -S atom site S(001) 1.0 Ni atom site 20 4.92 456 0,U=0V Bond site OOH 0.8 -Ring site Ni(001) 2.98 0.6 NiS(210) b200i) 0 S2(210) b4210) 0.4 R(210) 1.17 087 0.2 OH 0.52n 0 H'+e ⅓H -0.06 =1.23W 0.0 0.0 *+2H,0 02 -0.64 -0.64 02 -0.87 Reaction coordinate -2.32U=1.81V (210)surface (0.58) HER Mi111) ● 000m 0.91 OER 0.90 Ni/CeO-(111) 0.5 0.13 0 Ni4Ce0(111) Cu surface: 0.5 Ca02.(111) 1 H,O TS H+HO
Theoretical Calculations HER OER Electrocatalytic Water splitting
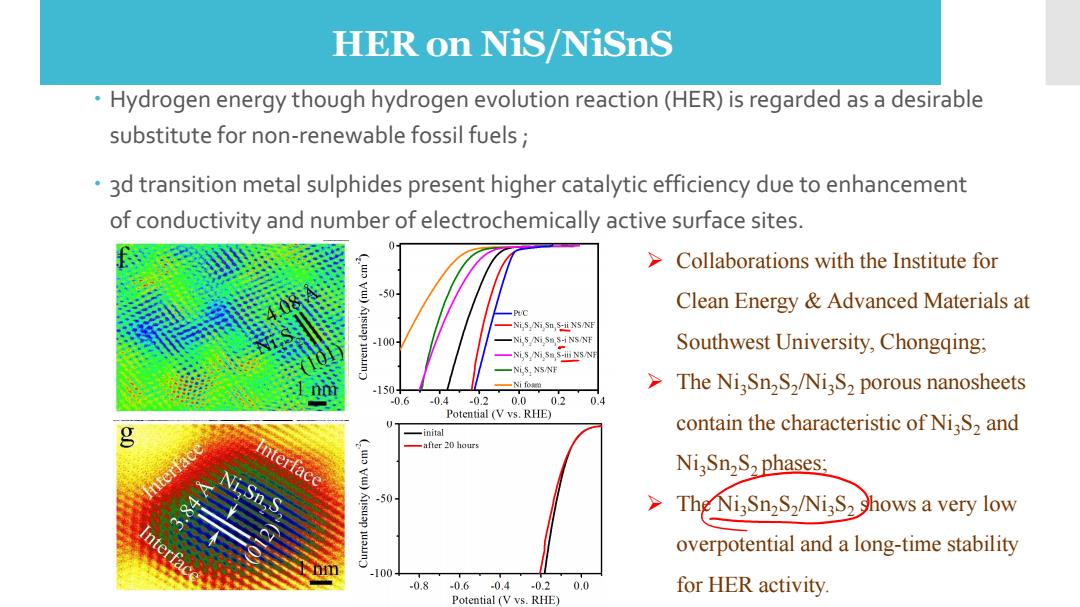
HER on NiS/NiSnS Hydrogen energy though hydrogen evolution reaction(HER)is regarded as a desirable substitute for non-renewable fossil fuels; 3d transition metal sulphides present higher catalytic efficiency due to enhancement of conductivity and number of electrochemically active surface sites. >Collaborations with the Institute for 50 Clean Energy Advanced Materials at -PC -Ni,S,Ni,Sn,S-ii N5/NF 100 -Ni,5 Ni,5n,S-i NS/NF -Ni,5 Ni,5m,5-ii NS/NF Southwest University,Chongqing; -NiS,NS/NE .150 -Nifo单 The Ni,Sn2S,/NiS,>porous nanosheets 0.6 -0.4 -0.2 0.0 02 0.4 Potential (V vs.RHE) -inital contain the characteristic of NiS,and Interface (wo vu) -after 20 hours Ni,Sn2S2 phases: -50 The NisSn2S2/NigS2 Shows a very low overpotential and a long-time stability -100 -0.8-0.6-0.4-0.20.0 for HER activity. Potential (V ys.RHE)
The catalytic activity of Ni3S2 and Ni2Sn3S2 toward hydrogen evolution reaction Hydrogen energy though hydrogen evolution reaction (HER) is regarded as a desirable substitute for non-renewable fossil fuels ; 3d transition metal sulphides present higher catalytic efficiency due to enhancement of conductivity and number of electrochemically active surface sites. ➢ Collaborations with the Institute for Clean Energy & Advanced Materials at Southwest University, Chongqing; ➢ The Ni3Sn2S2 /Ni3S2 porous nanosheets contain the characteristic of Ni3S2 and Ni3Sn2S2 phases; ➢ The Ni3Sn2S2 /Ni3S2 shows a very low overpotential and a long-time stability for HER activity. HER on NiS/NiSnS
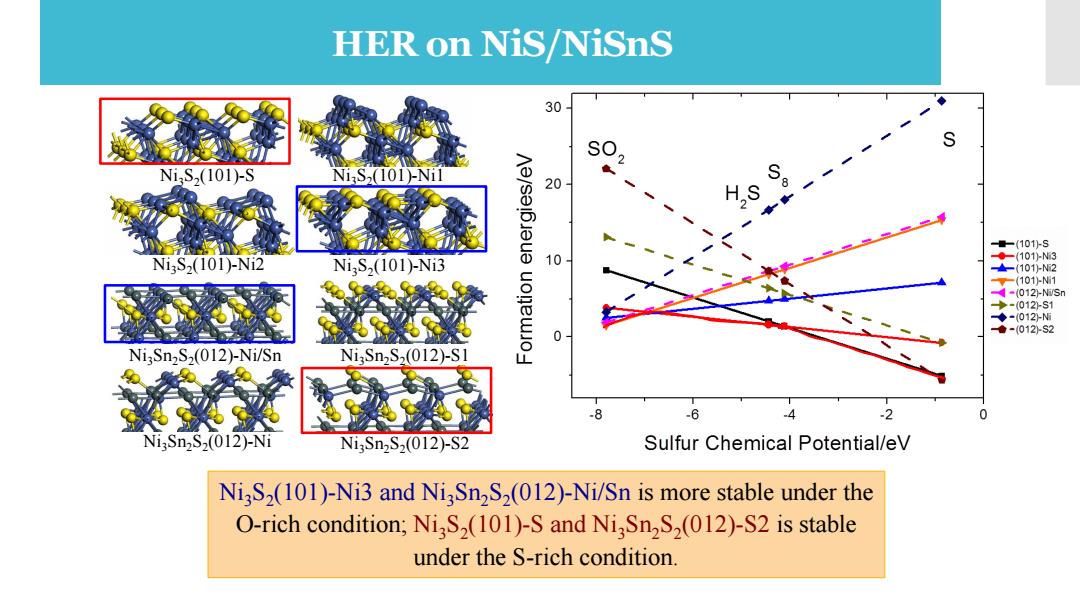
HER on NiS/NiSnS 30 NiS2(101)-S NiaS2(101)-Ni 20 量-(101)S Ni3S2(101)-Ni2 10 ◆(101-N3 Ni,S,(101)-Ni3 101-N2 -(101)1 4-(012-NSr ◆-(012外-S1 ◆-012分5 ●-(012外2 NiSn2S2(012)-Ni/Sn NiSn2S2(012)-S1 L .8 -6 4 2 NigSn2S2(012)-Ni Ni3Sn2S2(012)-S2 Sulfur Chemical Potential/eV NigS2(101)-Ni3 and NiSn2S2(012)-Ni/Sn is more stable under the O-rich condition;NiS2(101)-S and NiSn2S2(012)-S2 is stable under the S-rich condition
The catalytic activity of Ni3S2 and Ni2Sn3S2 toward hydrogen evolution reaction Ni3S2 (101)-Ni3 and Ni3Sn2S2 (012)-Ni/Sn is more stable under the O-rich condition; Ni3S2 (101)-S and Ni3Sn2S2 (012)-S2 is stable under the S-rich condition. Ni3S2 (101)-S Ni3S2 Ni3S2 (101)-Ni2 (101)-Ni3 Ni3S2 (101)-Ni1 Ni3Sn2S2 (012)-Ni/Sn Ni3Sn2S2 (012)-S1 Ni3Sn2S2 (012)-Ni Ni3Sn2S2 (012)-S2 HER on NiS/NiSnS