
04-Cancer immunotherapy Cancer immunotherapy From Wikipedia,the free encyclopedia Cancer imm erapy (m-onor totrea cancer Immunoth passive or hybrid(active and passive).These approaches exploit the fact that cancer cells often have molecules on heir sur e that can be det Dy th often proteins or other macromolecules (e carbohydrates).Active immunotherapy directs the immune G system【o attack tu mor cells by targeting TAAs.Passive s en and cytokines. Among these rapies system that bind to a tar omemgmeotTeehodiesrtproeciipovCeSeoane n pathogens.Each antibody is specific to one or a few proteins.Those that bind to tumor antigens treat cancer rtace receptors are com non targets I antibo ody therapies and ir lude CD20,CD27 and CD279.Once Approved antibodies include alemtuzumab,iplimumab,nivolumab,ofatumumab and rituximab. Active cellular th ual of in mune cells from the blood or fro r.Thos immune cells can be genetically engineered to express a tumor-specific receptor,cultured and returned to the patient.Cell types t be use in this v cells,lymphokin e-activated killer cells -approved cell-based therapy is Dendren's Provenge for th Interleukin-and in a are cytokin regula e and coordinate the behaviour of the immune ed in the tre related Ka ci'c ma,follicular lymphoma,chronic myeloid leukaemia and malignant melanoma.Interleukin-2 is used in the treatment of malignant melanoma and renal cell carcinoma. Contents 3 Antibody therapy dy types 3.3 FDA-app .5 Combination immunotherapy .6 Combinatorial ablation and immunotherapy
04-Cancer immunotherapy

。7 Polysaccharide-K ·8 Research .8.3 Anti-GD2 antibodies History oy for th r has bee nt of infection in the desired location and cause regression of the tumour.In 13th century,St.Peregrine experienced spontaneous regression of tumor,after the tumor became infected.In the 18th and 19th centuries,deliberate were a stand wounds were left ope French physician Dusso y so o品洁n的 posing the tumor to infection includin there sea relationship between infection and cancer regression date back to at least the 1th Modern Immunotherapy began in 1796 when Edward Jenner produced the first vaccine involving immunisation with cowpox to prevent smallpox.Towards the end of the 19th century Emil von Behring and Shibasaburo Kitasato discovered that injecting animals with diphtheria toxin produced blood serum with antitoxins to i provoking ar acioraphgtobadeia.h1968apmoleinrehidohiseg was identified and calle in prod rapy for cancer was introduced by Ste 厂oc or regres ate (2. 33%)in 1205 patients with metastatic cancer who underwent In 1987,rese archers identified cytotoxic T-lymphocyte antigen 4,or CTLA-4.Allison found that CTLA-4 prevents cks tumo 00 CTLA WOU the immune rs in In 1999 b ch firm Med hts to the dy In 2010 Bristol-Myers Squibb,who acquired Medarex in 2009,reported that patients with metastatic melanoma lived ded life in advanced melanoma in a randomized trial.st tme any treatme an average of 10 months on the antibody,versus 6 month: In the early 1990s,a biologist discovered a molecule expressed in dying T cells,which he called programmed bler of T cells.An antibody that targeted PD-1 was remission in multiple subjects across multiple cancer types.In 2013

In 1997 rituximab,the first antibody homa Since this a alemtuzumab (2001). ofatumumab (2009)and ipilimumab (2011). n2002 电5eo部amdm9 ously adminis traction with appropriate stimulatory cytokines. with both nti-CTIA-4 and anti-PD-1 一oo西some时 later.Son atients kep onding after the antibody had been di prostate The first cell-ba rapy cancer vaccine,sipuleucel-T,was approved in 2010 for the After success harves cells ,expanding them in the lab and reinfusing them This technique is a personalized treatment that involves genetically modifying each patient'scetota cllsuc e remission nriuhu By mid 2016 the FDA had approved one PD-L1 inhibitor(atezolizumab)and two PD-1 inhibitors (nivolumab and pembrolizumab). Cellular immunotherapy Dendritic cell therapy Dendritic cell therapy provokes anti-tumor responses by causing dendritic cells to present tumor antigens to lymphocytes,which m,priming them to other cells that pres the antigen ce APCS) f the immune system id igens is by Blood cells are tem oved omthe body PpaCepOeare'ofemgemnconmbiaionwithajvantshighly immunogenic substances)to increase the immune and anti-tumor responses.Other adjuvants include prote ns or oth r chemicals tha (GM-CSP) such as granulocyte macrophage be ach virus that expres sses GM-CSE aoacolyb dendritic cells f outside the peptide/protein or a tumor cell lysate (asolution of broken down tumor cells).These cells(with optional adjuvants)are infused and provoke an immune response

Dendritic cell therapies include the use of antibodies that bind to receptors on the surface of dendritic cells T6nmdm出e Sipuleucel-T nting cells from blood by leukapheresis and growing them with the fusion protein PA2024 高9 po Antibody therapy ibrboin oad playing a centr 八慧要 against specific antigens,such as those present on tumor surfaces. ”电 Antibody types Many forms of antibodies can be Conjugation Two typesare used in cancer treatments:4] Nakdnbdiesreibdehouadded elemeMost aibody therapesusehis Conjugated monoconaantibodies are joined to another moleue which is either cytotoxicor radioa tive. chemicals are tho e typically used as chemotherapy drugs,but other can be d-linked antbo immunotoxins antibodies are tagged with chemotherapeutic molecules or toxins,respectively.5 Human/non-human balance ,chimeric,humanized and human.Murine antibodies are from of th meric andin bodies rine aas the constant region.Humanized antibodies are almost completely human:only the complementarity determining o the varlable regions are derived fom mrine sources.Human antbodes have compleely human Cell death mechanisms Antibody-dependent cell-mediated cytotoxicity(ADCC) Antibody-dependent cell-mediated cytotoxicity (ADCC)reguires antibodies to bind to target cell surfaces. Antibodies are formed of a binding region(Fab)and the Fc region that can be detected by immune system cells pto und on many immun sys m c yme B to kill the or cell.E umab
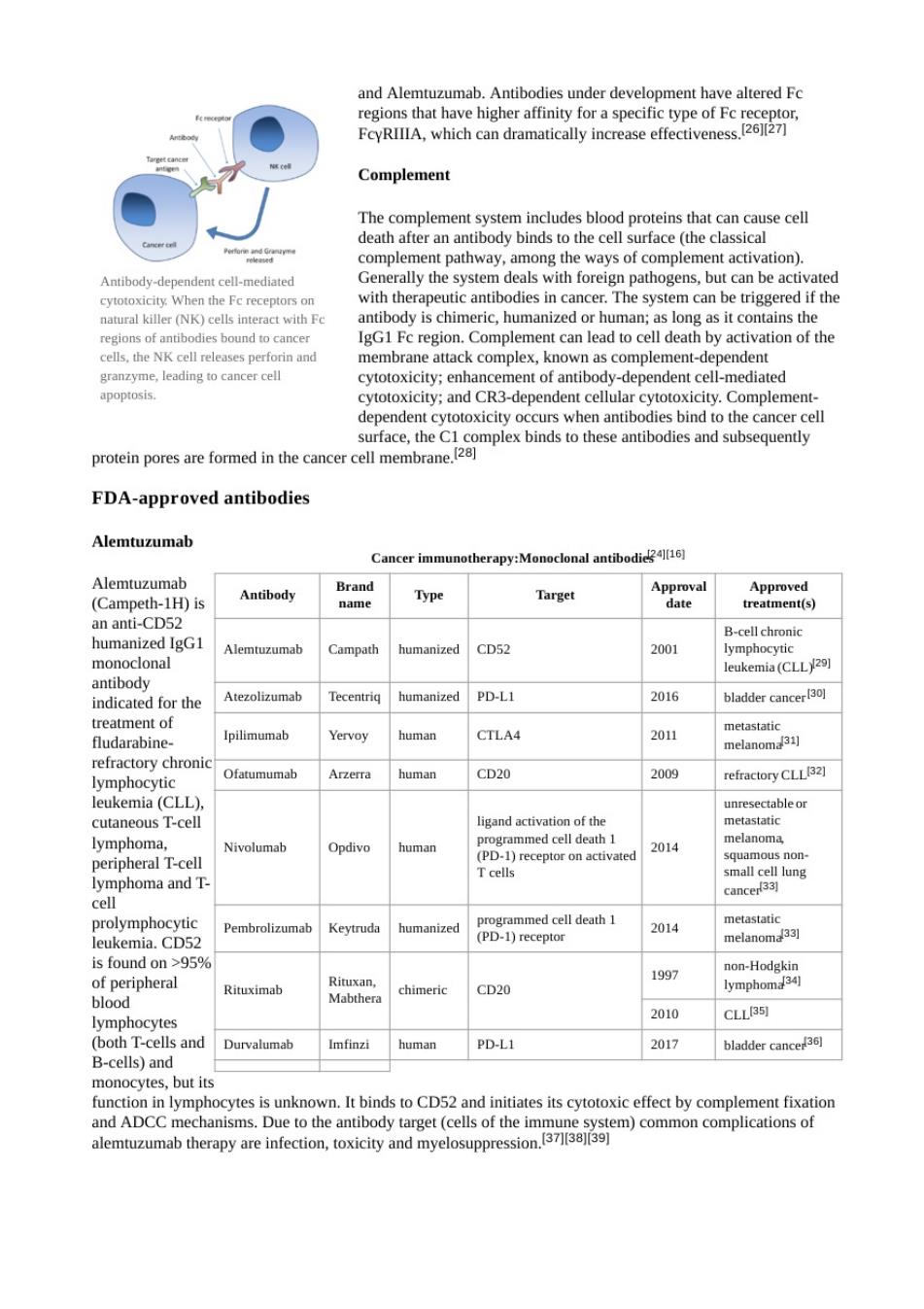
and alemtuzumab.antibodies under development have altered fc 将m代 Complement complement pathway,among the ways of complement activation). Antibody- ndent cell-mediated When the Ferecep Generally the systemr pathogens,but can be act the is chi ies in cance The system can be IgG1 Fc region.Com cells,the NK cel releses perforinand membrane attack complex,known as complement-dependent granzyme eadng tocancer cel r cytotox proeomplex binds to these antibodies and subseqny FDA-approved antibodies Alemtuzumab Cancer immunotherapy:Monoclonal antibodi Alemtuzu (Cam eth-1H)is Antibody Brand Type Approval m) an anti-CD52 humanized IgG1 CD52 2001 mo leukemia (CLL29) indicated for the Atezolizumab humanized PD-LI 2016 bladder cance treatment of fludarabine Ipilimumab Yervoy human CTLA4 2011 refractory chroni Ofatumumal human CD20 2009 refractory CLLB2] 1 eukemia(CLL】 cutaneous T-cell lymphoma. Nivolumab Opdivo human 2014 mall cell lur Pembrolizumab 2014 leukemia.CD5. 95 197 non-Hodgkin Rituximab Mabthera chimeric CD20 lymphom 2010 CLLB51 lymphocytes T-cells and Durvalumab Imfinzi human PD-L1 2017 bladder cancer3同 ells)an oyeiskIbinds to CD52dtcyotoxiefect by come ixio aeoaa