
Electrode Potentials: Cell potential is difference between anode and cathode potential Ecell =Ecathode-Eanode when half-reactions written as reductions Example: 2AgCl(s)+H2(g)>2Ag(s)+2H++2CI- 2AgCI(s)+2e>2Ag(s)+2CI- 2Ht+2e←→H2(g) electrons on left Galvanic cell Ecell=Ecathode-Eanode=+0.46 V Can't measure potential on each electrode independently -only differences CEM 333 page 10.6
Electrode Potentials: • Cell potential is difference between anode and cathode potential Ecell = Ecathode - Eanode when half-reactions written as reductions Example: 2AgCl(s) + H2 (g) ® 2Ag(s) + 2H+ + 2Cl- 2AgCl(s) + 2e- « 2Ag(s) + 2Cl- 2H+ + 2e- « H2 (g) electrons on left Galvanic cell Ecell=Ecathode-Eanode=+0.46 V Can't measure potential on each electrode independently - only differences CEM 333 page 10.6
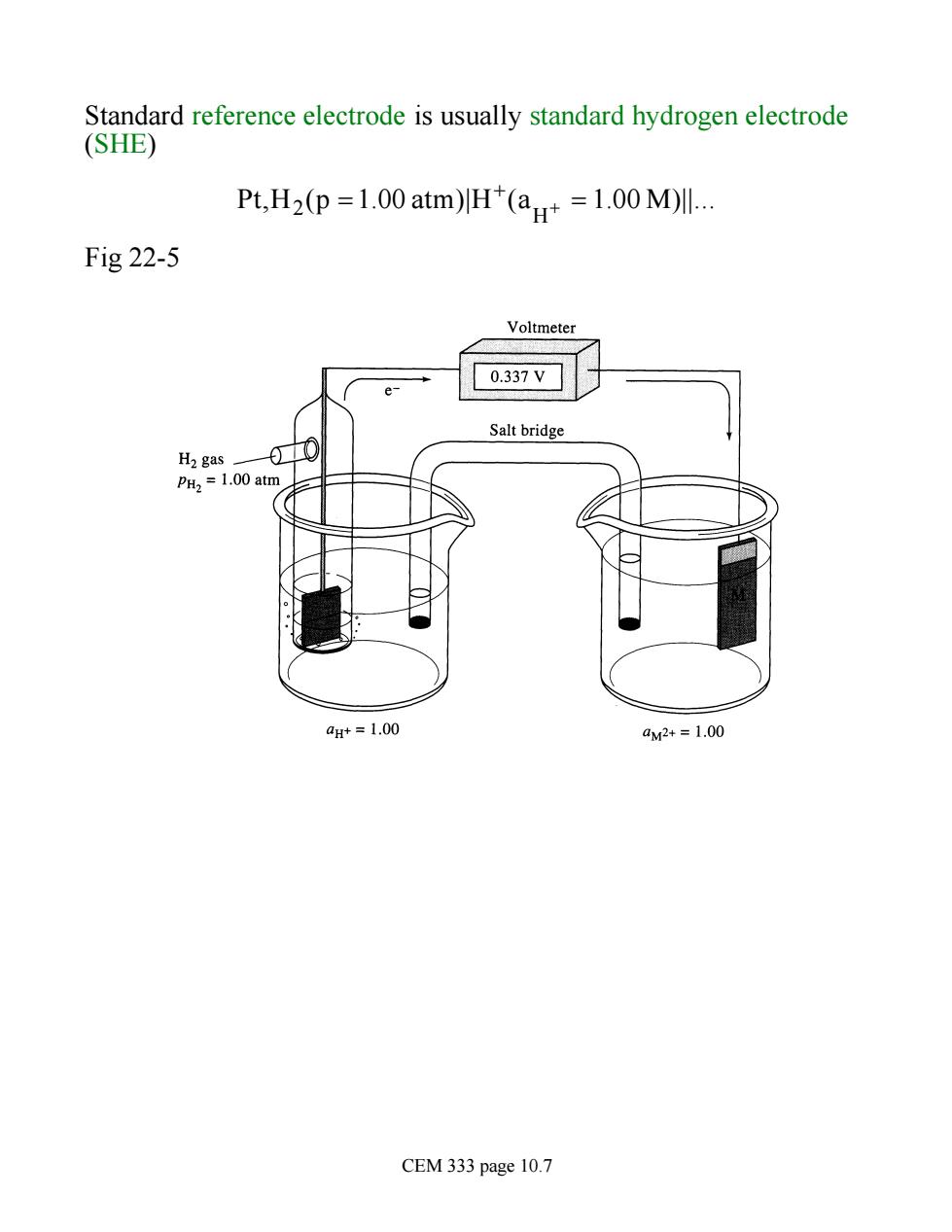
Standard reference electrode is usually standard hydrogen electrode (SHE) Pt,H2(p=1.00 atm)H(a=1.00 M)Il. Fig 22-5 Voltmeter e- 0.337V Salt bridge H2 gas 0 PH2=1.00 atm aH+=1.00 aw2+=1.00 CEM 333 page 10.7
Standard reference electrode is usually standard hydrogen electrode (SHE) Pt,H2 (p =1.00 atm)|H+ (aH + = 1.00 M)||. Fig 22-5 CEM 333 page 10.7