
Structures of Some Common Functional Groups (continued) Name Structure* Name ending Example Sulfide sulfide CHgSCH3 Dimethyl sulfide Disulfide disulfide CH3SSCH3 Dimethyl disulfide Sulfoxide sulfoxide 0 CH3SCH3 Dimethyl sulfoxide Aldehyde -al 0 CH3CH Ethanal Ketone -one CH3CCH3 Propanone Carboxylic acid -oic acid CH3COH Ethanoic acid

Ester -oate 0 CH3COCH3 Methyl ethanoate Thioester 。 -thioate 0 CH3CSCH3 Methyl ethanethioate Amide -amide CH3CNH2 Ethanamide Acid chloride oyl chloride 0 CH3CCI Ethanoyl chloride *The bonds whose connections aren't specified are assumed to be attached to carbon or hydrogen atoms in the rest of the molecules

林 0 Carboxylic acid a则 b) functional group C H3C-CH3 HgC OH Fig.1.(a)Ethane;(b)ethanoic acid. A0 Fig.2.(a)Aromatic ester,(b)aliphatic ester;(c)aromatic amide;(d)aliphatic amide
College of Science
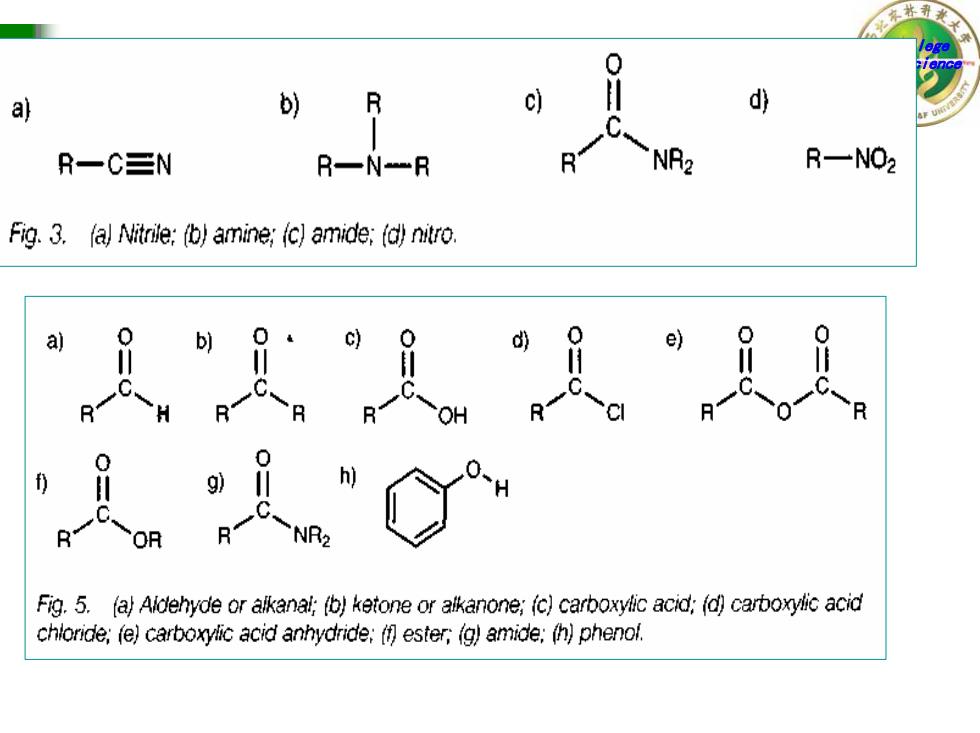
0 a b)R d R一C三N R一N一R NR2 R一NO2 Fig.3.(a Nitrile:(b)amine;(c)amide;(d)nitro. a b) e) H C-R h C NR2 Fig.5.(a)Aldehyde or alkanal;(b)ketone or alkanone;(c)carboxylic acid;(d)carboxylic acid chloride;(e)carboxylic acid anhydride:(f ester,(g)amide;(h)phenol
College of Science

a 0 b) c) CO2H CH3 CO2CH2CHg Fig.1.(a)Aliphatic ketone;(b)aliphatic ester;(c)aromatic carboxylic acid;(d)aromatic ketone. 0 CH CH Fig.2.(a)Aromatic ester;(b)aliphatic ester;(c)aromatic amide;(d)aliphatic amide
College of Science