
Nomenclature of Saturated Hydrocarbons
Nomenclature of Saturated Hydrocarbons
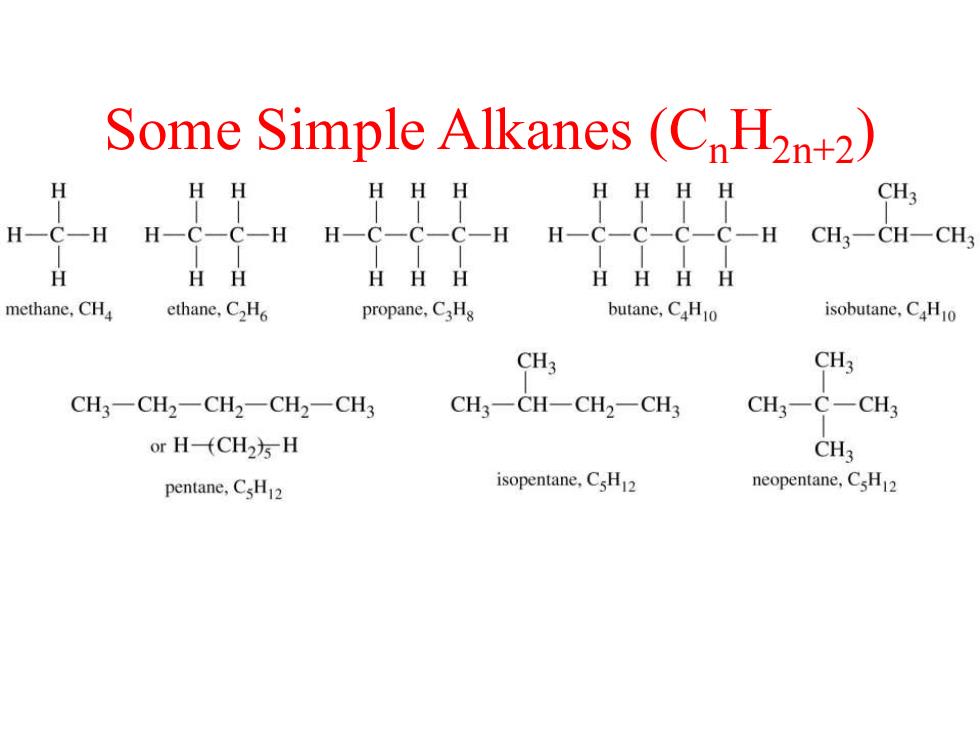
Some Simple Alkanes(C H2n+2) HH HHH CH3 H一C-HH-C-C-H H-C-C-C-H CH3一CH一CH H HH HHH HHH H methane,CHa ethane,C2H propane,CHg butane,CHo isobutane,CHo CH3 CH3 CH3-CH2一CH2-CH2-CH3 CH3-CH-CH2-CH3 CH3-C-CH3 or H-(CH2方H CH3 pentane,CsH2 isopentane,CsH2 neopentane,CsH12
Some Simple Alkanes (CnH2n+2)

The n-alkanes CH4 methane C2H6 ethane C3Hg propane C1H24 undecane C4H10 butane C12H26 dodecane C5H12 pentane C13H28 tridecane C6H14 hexane C14H30 tetradecane C7H16 heptane CIsH32 pentadecane C8H18 octane CoH20 nonane C10H22 decane
The n-alkanes C H4 C2H6 C3H8 C4H10 C5H12 C6H14 C7H16 C8H18 C9H20 C10H22 methane ethane propane butane pentane hexane heptane octane nonane decane C11H24 undecane C12H26 dodecane C13H28 tridecane C14H30 tetradecane C15H32 pentadecane

Boiling points 400 300 CH3-(CH2)n一CHg ()uod 3u!!oq 200 n-alkanes 100 CH3 0 CH-(CH2)CH3 -100 isoalkanes CH3 -200 0 5 10 15 20 number of carbon atoms
Boiling points
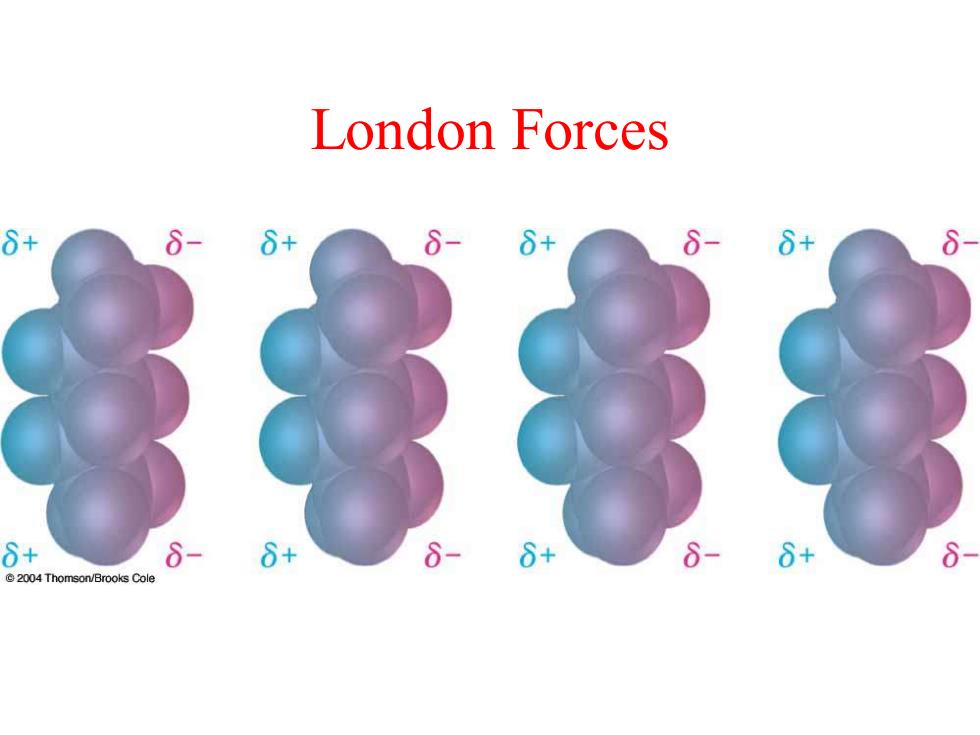
London Forces 8+ 8-6+ 8-8+ 6-6+ 8 8+ 6+ 6- 6+ 6+ @2004 Thomson/Brooks Cole
London Forces