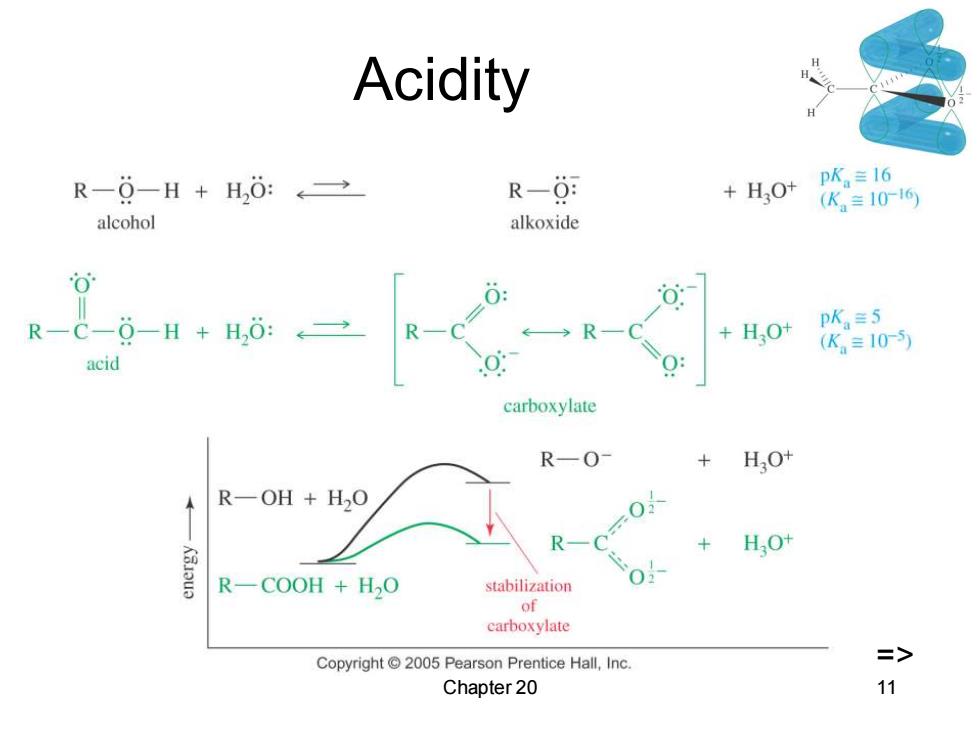
Acidity R-0-H+H,0:←→ R-0 +H,0 PK。≡I6 (K。=1016 alcohol alkoxide 0 R-C-0-H+H,0:←≥ ←→RC + HO+ pK≡5 (K。=10-5 acid carboxylate R-O- HO+ R一OH+H2O 0-0X R + HO+ R一COOH+HO stabilization 0 of carboxylate Copyright2005 Pearson Prentice Hall,Inc. => Chapter 20 11
Chapter 20 11 Acidity =>

Resonance Stabilization H H H => Copyright2005 Pearson Prentice Hall.Inc. 12
Chapter 20 12 Resonance Stabilization =>

Substituent Effects on Acidity H O CI -0-H 0-0-H CI- C一O-H CI-0 H CI acetic acid chloroacetic acid dichloroacetic acid trichloroacetic acid pK=4.74 pK,=2.86 pK=1.26 pK=0.64 stronger acids COOH COOH COOH COOH COOH NO2 NO2 OCH3 NO2 p-methoxy benzoic acid m-nitro p-nitro o-nitro => pKa= 4.46 4.19 3.47 3.41 2.16 13
Chapter 20 13 Substituent Effects on Acidity =>