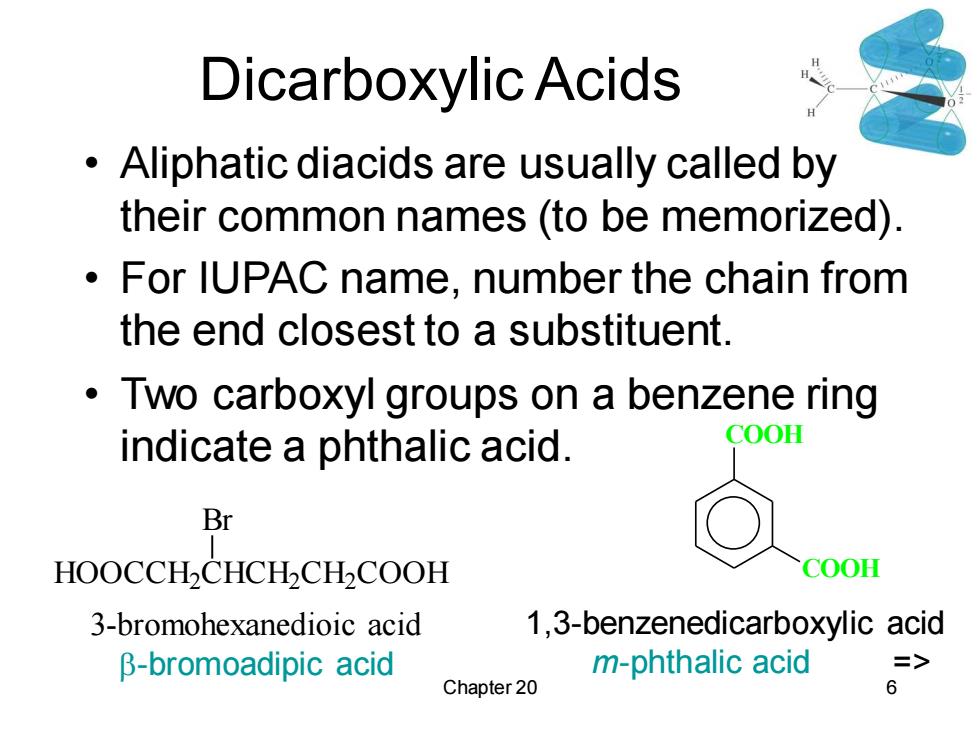
Dicarboxylic Acids Aliphatic diacids are usually called by their common names (to be memorized). For IUPAC name,number the chain from the end closest to a substituent. Two carboxyl groups on a benzene ring indicate a phthalic acid. COOH Br HOOCCH2CHCH2CH2COOH COOH 3-bromohexanedioic acid 1,3-benzenedicarboxylic acid β-bromoadipic acid m-phthalic acid => Chapter 20 6
Chapter 20 6 Dicarboxylic Acids • Aliphatic diacids are usually called by their common names (to be memorized). • For IUPAC name, number the chain from the end closest to a substituent. • Two carboxyl groups on a benzene ring indicate a phthalic acid. HOOCCH2 CHCH2 CH2 COOH Br 3-bromohexanedioic acid -bromoadipic acid COOH COOH 1,3-benzenedicarboxylic acid m-phthalic acid =>
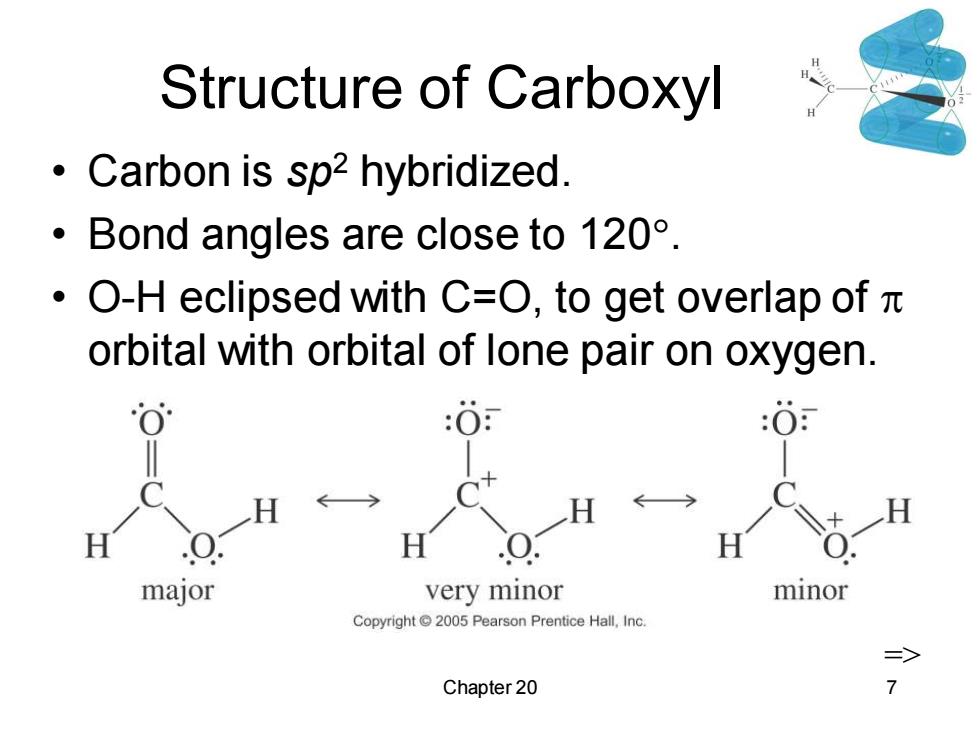
Structure of Carboxyl Carbon is sp2 hybridized. ·Bond angles are close to120°. ·O-H eclipsed with C=O,to get overlap ofπ orbital with orbital of lone pair on oxygen. :O :0 major very minor minor Copyright 2005 Pearson Prentice Hall,Inc. Chapter 20
Chapter 20 7 Structure of Carboxyl • Carbon is sp2 hybridized. • Bond angles are close to 120. • O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. =>
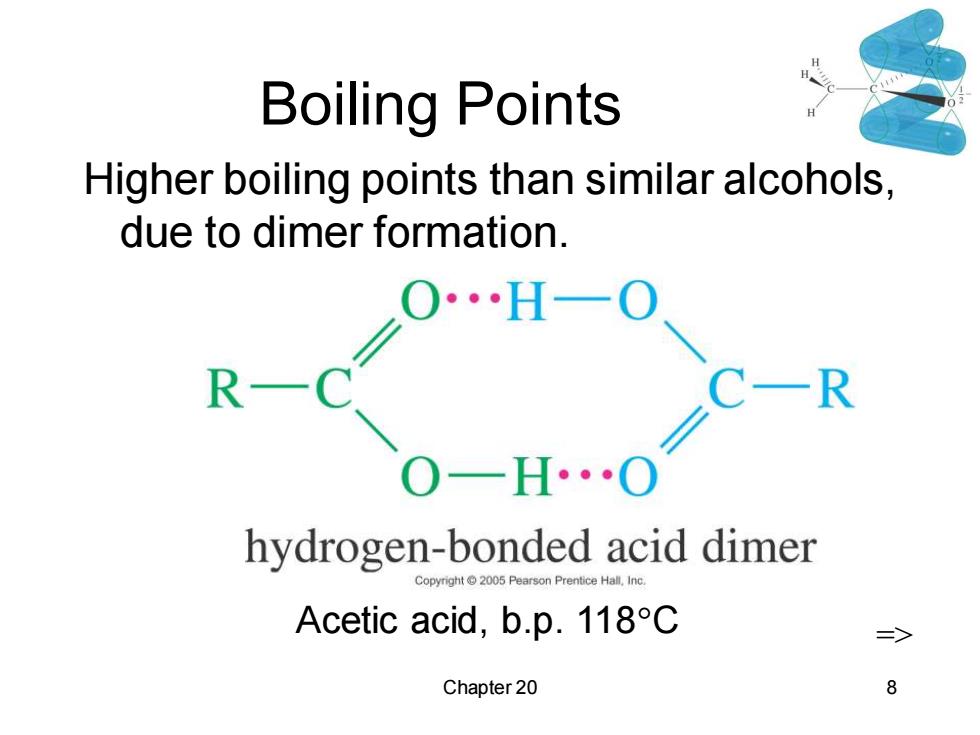
Boiling Points Higher boiling points than similar alcohols, due to dimer formation. C一R OH0 hydrogen-bonded acid dimer Copyright 2005 Pe on Prentice Hall,In Acetic acid,b.p.118C Chapter 20 8
Chapter 20 8 Boiling Points Higher boiling points than similar alcohols, due to dimer formation. Acetic acid, b.p. 118C =>

Melting Points Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis)lower the melting point.Note these 18-C acids: >Stearic acid (saturated):72C >Oleic acid (one cis double bond):16C >Linoleic acid (two cis double bonds):-5C => Chapter 20 9
Chapter 20 9 Melting Points • Aliphatic acids with more than 8 carbons are solids at room temperature. • Double bonds (especially cis) lower the melting point. Note these 18-C acids: ➢Stearic acid (saturated): 72C ➢Oleic acid (one cis double bond): 16C ➢Linoleic acid (two cis double bonds): -5C =>

Solubility Water solubility decreases with the length of the carbon chain. Up to 4 carbons,acid is miscible in water. More soluble in alcohol. Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. Chapter 20 10
Chapter 20 10 Solubility • Water solubility decreases with the length of the carbon chain. • Up to 4 carbons, acid is miscible in water. • More soluble in alcohol. • Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. =>