
Chapter 1 Biochemistry:An Evolving Science Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below.Not all of the answers will be used. a)uridine b)true c)AG d)thymine e)AH f)sugar-phosphate units g)covalent h)Archaea i)entropy j)system k)3 1)2 m)false 1.DNA is made from the building blocks adenine,guanine,cytosine,and Ans:d Section:1.2 2.The DNA backbone is made from repeating Ans:f Section:1.2 The number of hydrogen bonds formed between A and T. Ans:I Section:1.2 4. The number of hydrogen bonds formed between G and C. Ans:k Section:1.2 5.The fundamental groups of organisms include Eukarya,Bacteria,and Ans:h Section:1.1
Chapter 1 Biochemistry: An Evolving Science Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below. Not all of the answers will be used. a) uridine b) true c) G d) thymine e) H f) sugar-phosphate units g) covalent h) Archaea i) entropy j) system k) 3 l) 2 m) false 1. DNA is made from the building blocks adenine, guanine, cytosine, and ____________. Ans: d Section: 1.2 2. The DNA backbone is made from repeating ____________. Ans: f Section: 1.2 3. ____________ The number of hydrogen bonds formed between A and T. Ans: l Section: 1.2 4. ____________ The number of hydrogen bonds formed between G and C. Ans: k Section: 1.2 5. The fundamental groups of organisms include Eukarya, Bacteria, and ____________. Ans: h Section: 1.1

Chapter 1 Biochemistry:An Evolving Science 2 6 The strongest bonds in molecules: Ans:g Section:1.3 7 Hydrogen bonds are usually stronger than covalent bonds(true/false). Ans:b Section:1.3 8 Matter within a defined region of space Ans:j Section:1.3 9 For spontaneous reactions the AG must be positive. Ans:m Section:1.3 10. Gibbs free energy. Ans:c Section:1.3 Multiple Choice Questions 11.The structure of DNA described by Watson and Crick included A) a double helix. B) the sugar phosphate backbone aligned in the center of the helix. C) the base pairs that are stacked on the inside of the double helix. D) a and b. E)a and c. Ans:E Section:1.2 12.What did Watson and Crick suggest to be significant about the base pairing found in the helix? A)It allowed the DNA to twist in a helix. B) The DNA could be circular. C It was a mechanism for copying. D) All of the above. E)None of the above. Ans:C Section:1.3
Chapter 1 Biochemistry: An Evolving Science 2 6. ____________ The strongest bonds in molecules: Ans: g Section: 1.3 7. ____________ Hydrogen bonds are usually stronger than covalent bonds (true/false). Ans: b Section: 1.3 8. ____________ Matter within a defined region of space. Ans: j Section: 1.3 9. ____________ For spontaneous reactions the G must be positive. Ans: m Section: 1.3 10. ____________ Gibbs free energy. Ans: c Section: 1.3 Multiple Choice Questions 11. The structure of DNA described by Watson and Crick included A) a double helix. B) the sugar phosphate backbone aligned in the center of the helix. C) the base pairs that are stacked on the inside of the double helix. D) a and b. E) a and c. Ans: E Section: 1.2 12. What did Watson and Crick suggest to be significant about the base pairing found in the helix? A) It allowed the DNA to twist in a helix. B) The DNA could be circular. C) It was a mechanism for copying. D) All of the above. E) None of the above. Ans: C Section: 1.3

Chapter 1 Biochemistry:An Evolving Science 13.Approximately what percentage of the human genome encodes for proteins? A) 50% B) 90% c) 10% D 3% E)None of the above Ans:D Section 1.4 14.What gives proteins such a dominant role in biochemistry? A)the variation in protein sizes B) the ability to act as a blueprint C)their ability to self-replicate D)their ability to spontaneously fold into complex three-dimensional structures E)All of the above. Ans:D Section:1.4 15.If the whole chain is used in a non-overlapping frame,how many amino acids are defined by this DNA sequence:ATGTTTGGACTA? A)four B)two C)twelve D)six E)three Ans:A Section:1.4 16.What is the H+]concentration in a urine sample that has a pH of 6? A)10-6M B) 10-8M C) 10°M D)10-14M E)8M Ans:B Section 1.3 17.Which of the following is considered as a noncovalent bond? A) electrostatic interactions D) All of the above. B) hydrogen bonds E) None of the above. C) van der Waals interactions Ans:D Section:1.3 18.The energies for hydrogen bonds are approximately A)400 kJ/mol. D 200 kJ/mol. B)100-240kJ/mol E) None of the above C)4-20 kJ/mol. Ans:C Section:1.3 19.What pairs of atoms in bases are involved in hydrogen bonds? A)N-H and O-H D) All of the above. B)N-H and S-H E) None of the above. C)O-H and P-O Ans:A Section:1.3
Chapter 1 Biochemistry: An Evolving Science 3 13. Approximately what percentage of the human genome encodes for proteins? A) 50% B) 90% C) 10% D) 3% E) None of the above. Ans: D Section 1.4 14. What gives proteins such a dominant role in biochemistry? A) the variation in protein sizes B) the ability to act as a blueprint C) their ability to self-replicate D) their ability to spontaneously fold into complex three-dimensional structures E) All of the above. Ans: D Section: 1.4 15. If the whole chain is used in a non-overlapping frame, how many amino acids are defined by this DNA sequence: ATGTTTGGACTA? A) four B) two C) twelve D) six E) three Ans: A Section: 1.4 16. What is the [H+ ] concentration in a urine sample that has a pH of 6? A) 10 -6 M B) 10 -8 M C) 10 6 M D) 10 -14 M E) 8 M Ans: B Section 1.3 17. Which of the following is considered as a noncovalent bond? A) electrostatic interactions D) All of the above. B) hydrogen bonds E) None of the above. C) van der Waals interactions Ans: D Section: 1.3 18. The energies for hydrogen bonds are approximately A) 400 kJ/mol. D) 200 kJ/mol. B) 100–240 kJ/mol. E) None of the above. C) 4–20 kJ/mol. Ans: C Section: 1.3 19. What pairs of atoms in bases are involved in hydrogen bonds? A) N—H and O—H D) All of the above. B) N—H and S—H E) None of the above. C) O—H and P—O Ans: A Section: 1.3

Chapter 1 Biochemistry:An Evolving Science 4 20.Typical van der Waals energies are about A) 4-20 kJ/mol D) All of the above. B) 2-4 kJ/mol. E) None of the above. C)200 kJ/mol. Ans:B Section:1.3 21.What two properties of water are important for biological interactions? A)the polarity of water D) a and c B)the density of water E) b and c C)the cohesive properties of water Ans:D Section:1.3 22.The First Law of Thermodynamics states A)diversity is the result of gradual evolution. B)the total entropy of a system and its surroundings always increases for a spontaneous process. C) the total energy of a system and its surroundings is constant. D) light is both particle and wave. E)None of the above. Ans:C Section:1.3 23.The Second Law of Thermodynamics states A)the total entropy of a system and its surroundings always increases for a spontaneous process. B) temperatures will always decrease. C) the total energy of a system and its surroundings is constant. D) diversity is the result of gradual evolution. E) None of the above. Ans:A Section:1.3 24.List atoms commonly found in biological molecules that are often hydrogen-bond acceptors. A)carbon B)oxygen C)nitrogen D)b and c E)All of the above. Ans:D Section:1.3 25.Enthalpy is defined as A)a spontaneous reaction. D) All of the above. B)the entropy of the system. E) None of the above. C)the heat content of a system. Ans:C Section:1.3 26.If a particular reaction has a negative AG,is it likely to occur? A)Not unless energy is added to the system. B)Yes,if it is coupled to another reaction. C)Yes,it is spontaneous. D)No,it will never occur. E)Yes,if it takes place within a constrained area. Ans:C Section:1.3
Chapter 1 Biochemistry: An Evolving Science 4 20. Typical van der Waals energies are about A) 4–20 kJ/mol. D) All of the above. B) 2–4 kJ/mol. E) None of the above. C) 200 kJ/mol. Ans: B Section: 1.3 21. What two properties of water are important for biological interactions? A) the polarity of water D) a and c B) the density of water E) b and c C) the cohesive properties of water Ans: D Section: 1.3 22. The First Law of Thermodynamics states A) diversity is the result of gradual evolution. B) the total entropy of a system and its surroundings always increases for a spontaneous process. C) the total energy of a system and its surroundings is constant. D) light is both particle and wave. E) None of the above. Ans: C Section: 1.3 23. The Second Law of Thermodynamics states A) the total entropy of a system and its surroundings always increases for a spontaneous process. B) temperatures will always decrease. C) the total energy of a system and its surroundings is constant. D) diversity is the result of gradual evolution. E) None of the above. Ans: A Section: 1.3 24. List atoms commonly found in biological molecules that are often hydrogen-bond acceptors. A) carbon B) oxygen C) nitrogen D) b and c E) All of the above. Ans: D Section: 1.3 25. Enthalpy is defined as A) a spontaneous reaction. D) All of the above. B) the entropy of the system. E) None of the above. C) the heat content of a system. Ans: C Section: 1.3 26. If a particular reaction has a negative G, is it likely to occur? A) Not unless energy is added to the system. B) Yes, if it is coupled to another reaction. C) Yes, it is spontaneous. D) No, it will never occur. E) Yes, if it takes place within a constrained area. Ans: C Section: 1.3
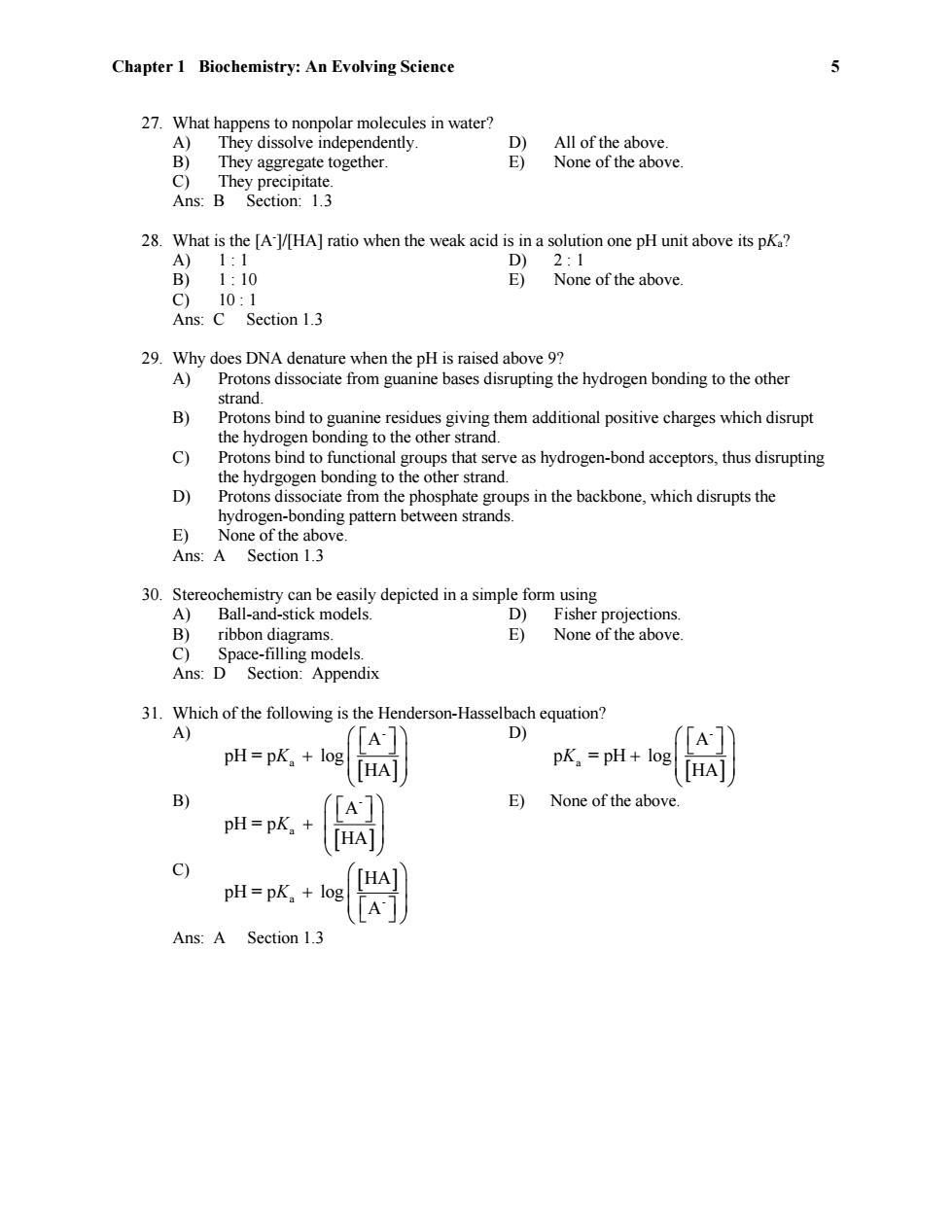
Chapter 1 Biochemistry:An Evolving Science 5 27.What happens to nonpolar molecules in water? A) They dissolve independently. D) All of the above. B) They aggregate together. E) None of the above. C)They precipitate. Ans:B Section:1.3 28.What is the [A-l/HA]ratio when the weak acid is in a solution one pH unit above its pKa? A)1:1 D) 2:1 B)1:10 E) None of the above. C)10:1 Ans:C Section 1.3 29.Why does DNA denature when the pH is raised above 9? A)Protons dissociate from guanine bases disrupting the hydrogen bonding to the other strand. B) Protons bind to guanine residues giving them additional positive charges which disrupt the hydrogen bonding to the other strand. c) Protons bind to functional groups that serve as hydrogen-bond acceptors,thus disrupting the hydrgogen bonding to the other strand. D) Protons dissociate from the phosphate groups in the backbone,which disrupts the hydrogen-bonding pattern between strands. E)None of the above. Ans:A Section 1.3 30.Stereochemistry can be easily depicted in a simple form using A) Ball-and-stick models. D)Fisher projections. B)ribbon diagrams. E)None of the above. C) Space-filling models. Ans:D Section:Appendix 31.Which of the following is the Henderson-Hasselbach equation? A) D) A pH=pK log pK。=pH+log HA HA B) E) None of the above. pH=pK. pH=pK。+ Ans:A Section 1.3
Chapter 1 Biochemistry: An Evolving Science 5 27. What happens to nonpolar molecules in water? A) They dissolve independently. D) All of the above. B) They aggregate together. E) None of the above. C) They precipitate. Ans: B Section: 1.3 28. What is the [A-]/[HA] ratio when the weak acid is in a solution one pH unit above its pKa? A) 1 : 1 D) 2 : 1 B) 1 : 10 E) None of the above. C) 10 : 1 Ans: C Section 1.3 29. Why does DNA denature when the pH is raised above 9? A) Protons dissociate from guanine bases disrupting the hydrogen bonding to the other strand. B) Protons bind to guanine residues giving them additional positive charges which disrupt the hydrogen bonding to the other strand. C) Protons bind to functional groups that serve as hydrogen-bond acceptors, thus disrupting the hydrgogen bonding to the other strand. D) Protons dissociate from the phosphate groups in the backbone, which disrupts the hydrogen-bonding pattern between strands. E) None of the above. Ans: A Section 1.3 30. Stereochemistry can be easily depicted in a simple form using A) Ball-and-stick models. D) Fisher projections. B) ribbon diagrams. E) None of the above. C) Space-filling models. Ans: D Section: Appendix 31. Which of the following is the Henderson-Hasselbach equation? A) - a A pH = p log HA K D) - a A p = pH log HA K B) - a A pH = p HA K E) None of the above. C) a - HA pH = p log A K Ans: A Section 1.3