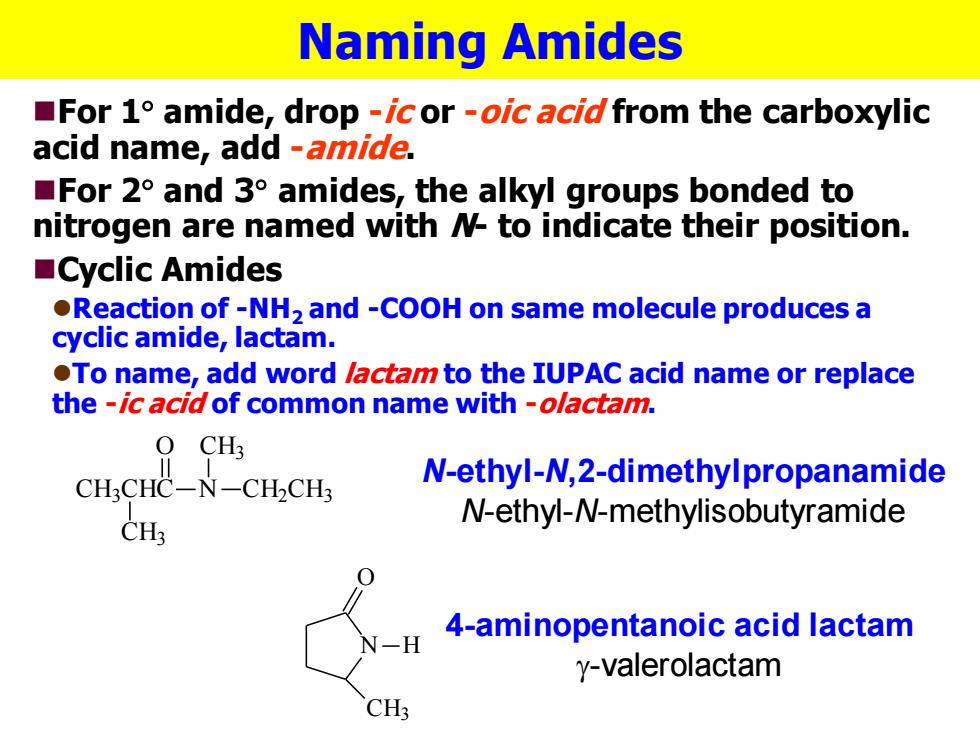
Naming Amides For 1 amide,drop -icor -oic acid from the carboxylic acid name,add -amide. For 2 and 3 amides,the alkyl groups bonded to nitrogen are named with N to indicate their position. ■Cyclic Amides Reaction of-NH2 and -COOH on same molecule produces a cyclic amide,lactam. To name,add word lactam to the IUPAC acid name or replace the -ic acid of common name with-olactam. O CH? CH3CHC-N-CH2CH3 N-ethyl-N,2-dimethylpropanamide CH; N-ethyl-N-methylisobutyramide 4-aminopentanoic acid lactam y-valerolactam CH3
Naming Amides ◼For 1 amide, drop -ic or -oic acid from the carboxylic acid name, add -amide. ◼For 2 and 3 amides, the alkyl groups bonded to nitrogen are named with N- to indicate their position. ◼Cyclic Amides ⚫Reaction of -NH2 and -COOH on same molecule produces a cyclic amide, lactam. ⚫To name, add word lactam to the IUPAC acid name or replace the -ic acid of common name with -olactam. CH3CHC N O CH2CH3 CH3 CH3 N-ethyl-N,2-dimethylpropanamide N-ethyl-N-methylisobutyramide N O CH3 H 4-aminopentanoic acid lactam g-valerolactam

N-alkylalkanamide (IUPAC:alkane name -e amide common:acid name -ic amide NH2 (propionamide) 丙酰胺 N-甲基乙酰胺 NH-CH3 (N-methylacetamide) N,N一二甲基甲酰胺 H N(CH3)2(N,N-dimethylformamide,DMF)
N-alkylalkanamide (IUPAC: alkane name - e + amide common: acid name - ic + amide) NH2 O O NH CH3 H O N(CH3 )2 (propionamide) (N-methylacetamide) (N,N-dimethylformamide, DMF) 丙酰胺 N-甲基乙酰胺 N,N-二甲基甲酰胺
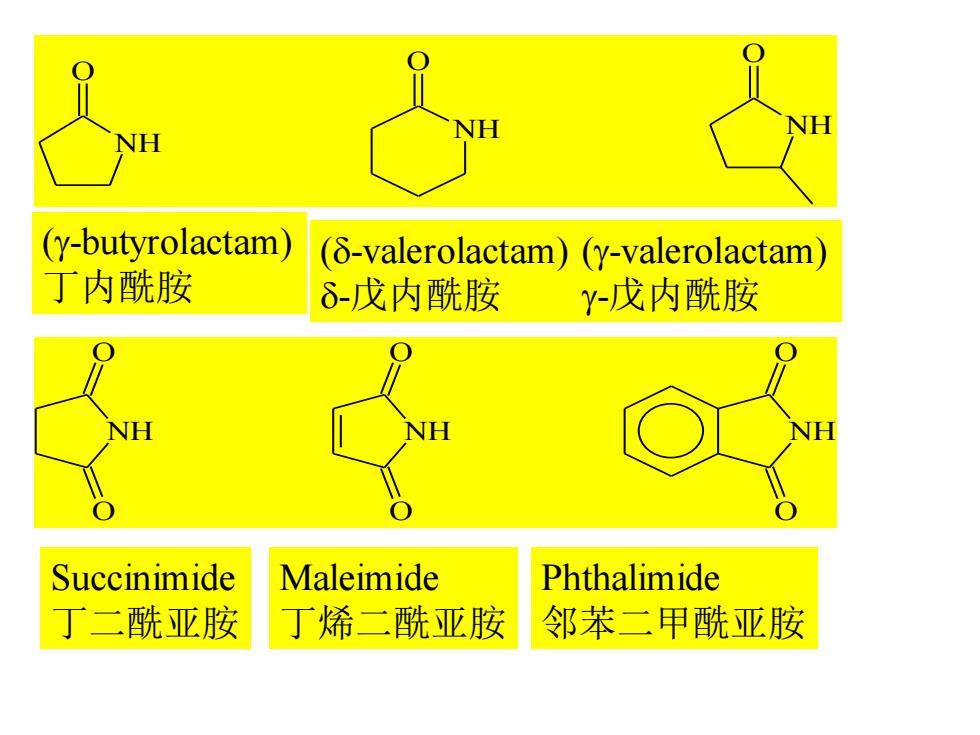
(y-butyrolactam) (8-valerolactam)(y-valerolactam) 丁内酰胺 δ-戊内酰胺 Y-戊内酰胺 Succinimide Maleimide Phthalimide 丁二酰亚胺 丁烯二酰亚胺 邻苯二甲酰亚胺
NH O NH O NH O (g-butyrolactam) 丁内酰胺 (d-valerolactam) d-戊内酰胺 (g-valerolactam) g-戊内酰胺 NH O O NH O O NH O O Succinimide 丁二酰亚胺 Maleimide 丁烯二酰亚胺 Phthalimide 邻苯二甲酰亚胺
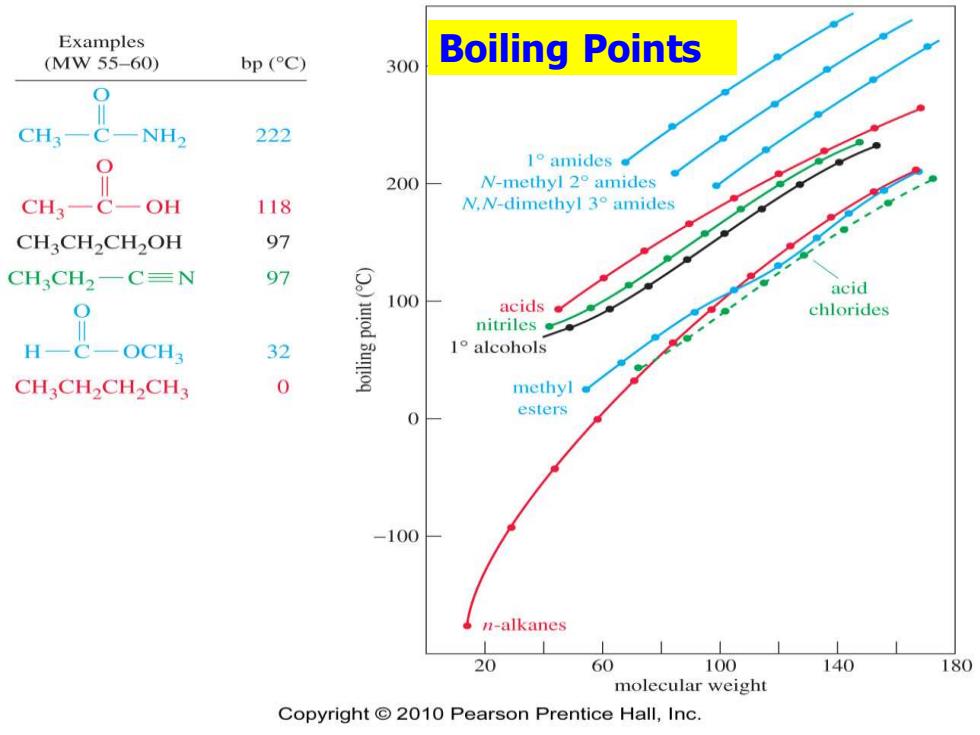
Examples (MW55-60) bp (C) 300 Boiling Points 0 CH3- NH2 222 O 1o amides 200 W-methyl2°amides CH3一C OH 118 N.N-dimethyl 3amides CHCH2CH2OH 97 CH3CH2一C=N 97 acid 100 acids chlorides nitriles OCH3 32 1°alcohols CHCH2CH2CH3 0 methyl esters 0 -100 n-alkanes 20 60 100 140 180 molecular weight Copyright 2010 Pearson Prentice Hall,Inc
Boiling Points
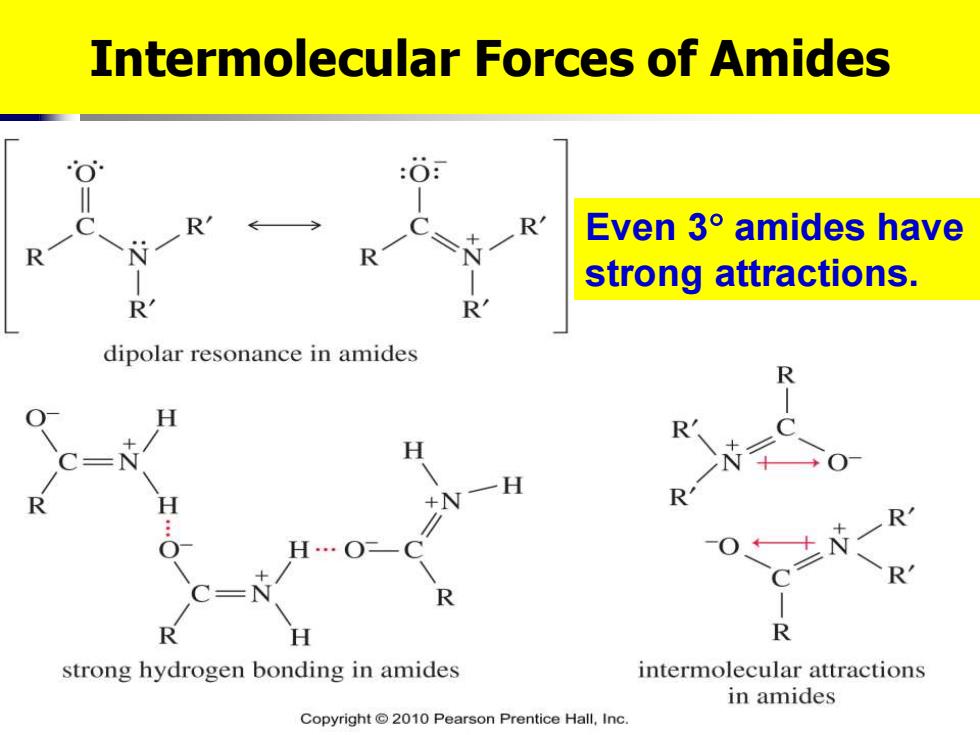
Intermolecular Forces of Amides 个 Even3°amides have strong attractions. R R dipolar resonance in amides R 一H R strong hydrogen bonding in amides intermolecular attractions in amides Copyright2010 Pearson Prentice Hall,Inc
Intermolecular Forces of Amides Even 3 amides have strong attractions