
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 38 Cengel Chapter 8 Exergy-A measure of work potential Spring,4/30/2019 Prof.,Dr.Yonghua HUANG 强 MAMMMAAN http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 38 Spring, 4/30/2019 Prof., Dr. Yonghua HUANG Cengel Chapter 8 Exergy – A measure of work potential http://cc.sjtu.edu.cn/G2S/site/thermo.html
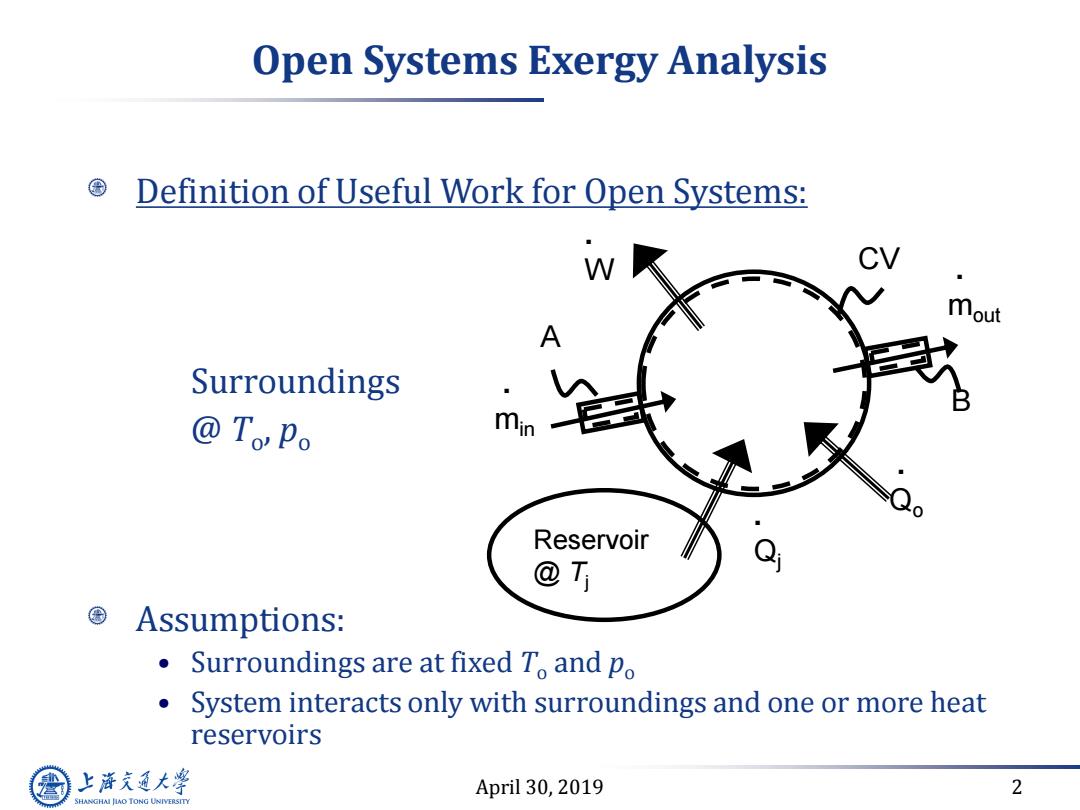
Open Systems Exergy Analysis Definition of Useful Work for Open Systems: W mout Surroundings @ToPo Reservoir @T Assumptions: ● Surroundings are at fixed To and po System interacts only with surroundings and one or more heat reservoirs 上游充通大 April 30,2019 2 SHANGHAI JLAO TONG UNIVERSITY
April 30, 2019 2 Open Systems Exergy Analysis Definition of Useful Work for Open Systems: Surroundings @ To , po Assumptions: • Surroundings are at fixed To and po • System interacts only with surroundings and one or more heat reservoirs . W . Qj . min . mout B A CV . Qo Reservoir @ Tj
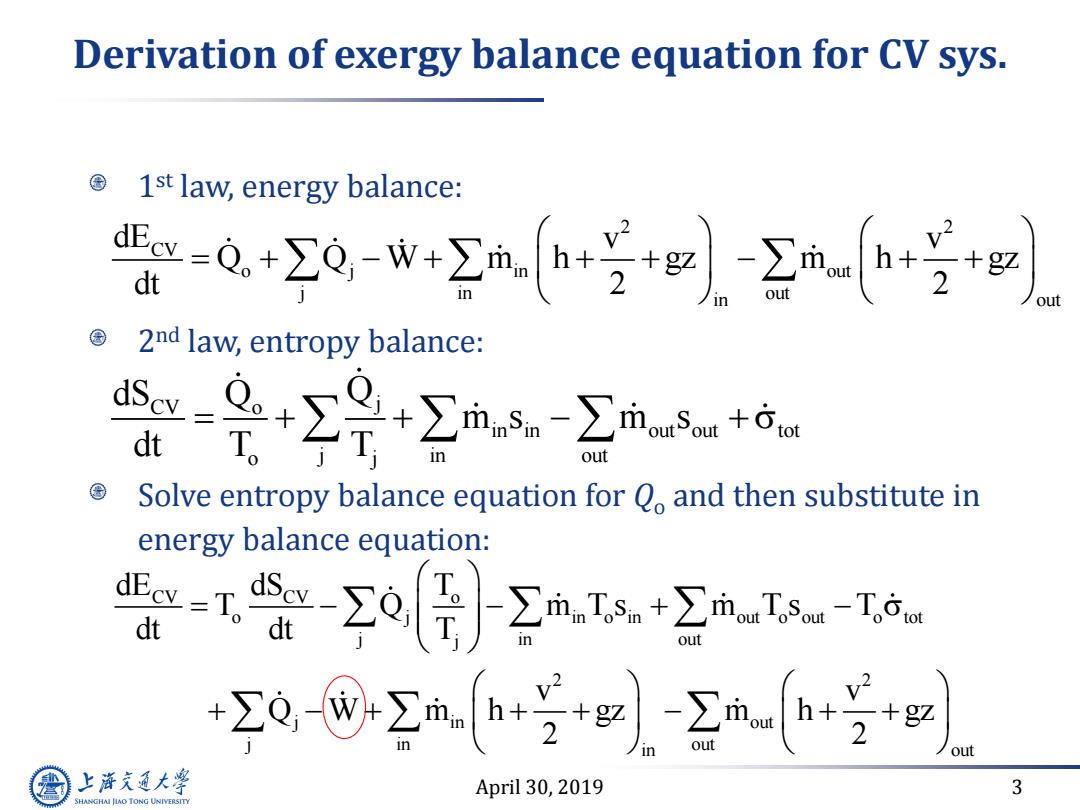
Derivation of exergy balance equation for CV sys. 1st law,energy balance: -0,w号 dt out 2nd law,entropy balance: -是+∑是+∑msm+ dt T。 out Solve entropy balance equation for Q,and then substitute in energy balance equation: -工o[aIs+2n- dt out +0-8mh++-2m+ +g2 out 2 out 上游通大学 April 30,2019 3 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 3 Derivation of exergy balance equation for CV sys. 1 st law, energy balance: 2 nd law, entropy balance: Solve entropy balance equation for Qo and then substitute in energy balance equation: 2 2 CV o j in out j in out in out dE v v Q Q W m h gz m h gz dt 2 2 CV o j in in out out tot o j j in out dS Q Q m s m s dt T T CV CV o o j in o in out o out o tot j in out j 2 2 j in out j in out in out dE dS T T Q m T s m T s T dt dt T v v Q W m h gz m h gz 2 2
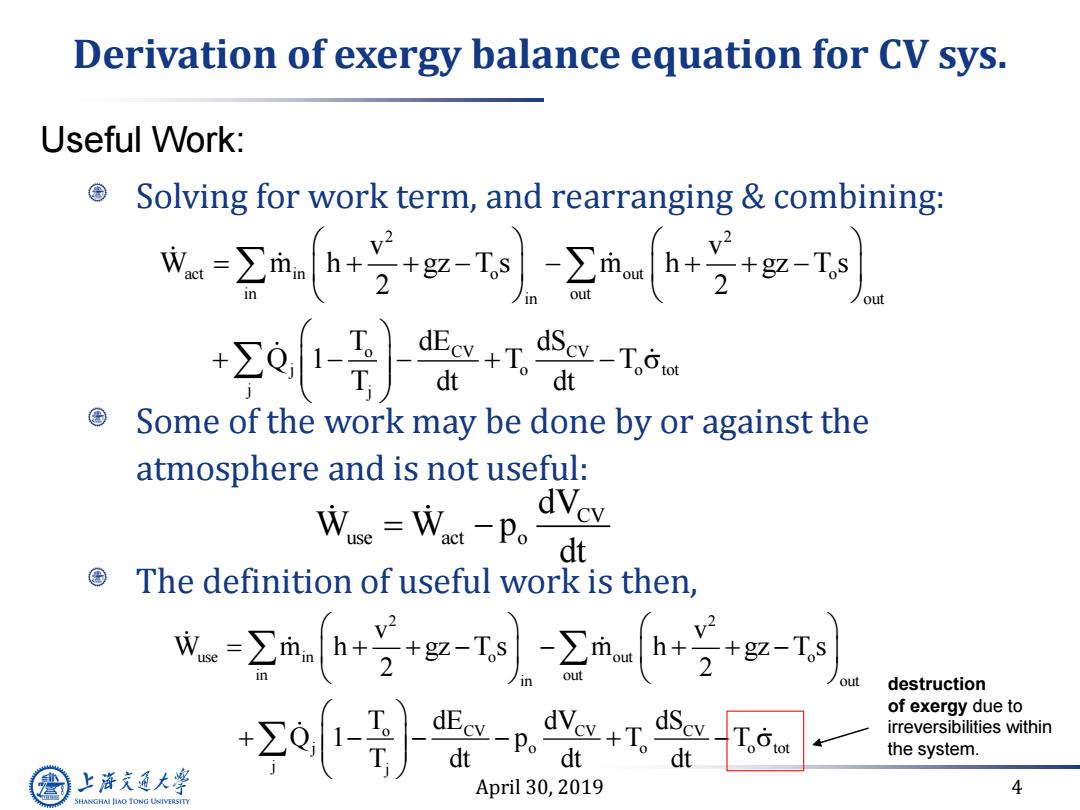
Derivation of exergy balance equation for CV sys. Useful Work: Solving for work term,and rearranging combining: w-m hg-ta mahst) +o-}监+会- dt Some of the work may be done by or against the atmosphere and is not useful: We=Wca-P。 dVev dt The definition of useful work is then, ma++g-T-mn+号g-t out destruction +-} dEcv-Po dScv of exergy due to irreversibilities within dt the system. 上游充通大学 April 30,2019 4 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 4 Derivation of exergy balance equation for CV sys. Solving for work term, and rearranging & combining: Some of the work may be done by or against the atmosphere and is not useful: The definition of useful work is then, 2 2 act in o out o in out in out o CV CV j o o tot j j v v W m h gz T s m h gz T s 2 2 T dE dS Q 1 T T T dt dt CV use act o dV W W p dt Useful Work: 2 2 use in o out o in out in out o CV CV CV j o o o tot j j v v W m h gz T s m h gz T s 2 2 T dE dV dS Q 1 p T T T dt dt dt destruction of exergy due to irreversibilities within the system
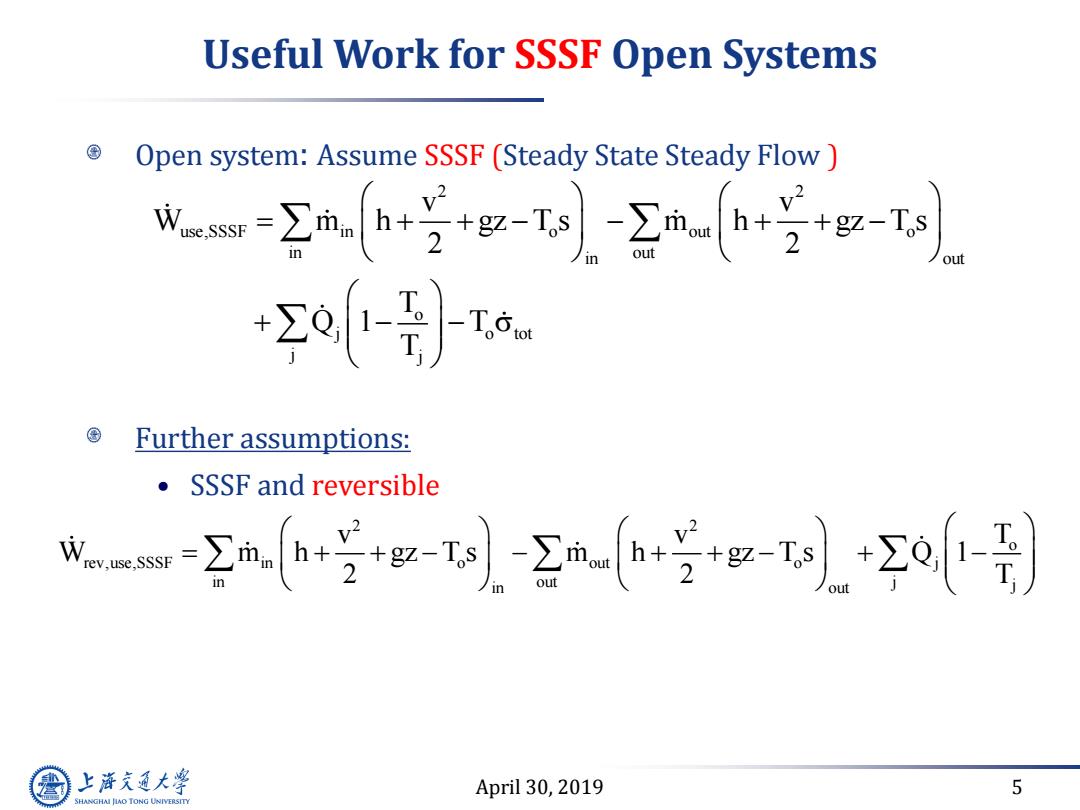
Useful Work for SSSF Open Systems Open system:Assume SSSF (Steady State Steady Flow 成-Σg-T-2h片+g ,v2 o-}- Further assumptions: ●SSSF and reversible wmm-空-g--2+5g以-2o-) 上游通大学 April 30,2019 5 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 5 Useful Work for SSSF Open Systems Open system: Assume SSSF (Steady State Steady Flow ) Further assumptions: • SSSF and reversible 2 2 use,SSSF in o out o in out in out o j o tot j j v v W m h gz T s m h gz T s 2 2 T Q 1 T T 2 2 o rev,use,SSSF in o out o j in out j j in out v v T W m h gz T s m h gz T s Q 1 2 2 T