上岸充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 32 Chapter 7 Entropy (Section 7.13) Spring,4/18/2019 强 Prof.,Dr.Yonghua HUANG A月L是。 http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 32 Spring, 4/18/2019 Prof., Dr. Yonghua HUANG Chapter 7 Entropy (Section 7.13) http://cc.sjtu.edu.cn/G2S/site/thermo.html
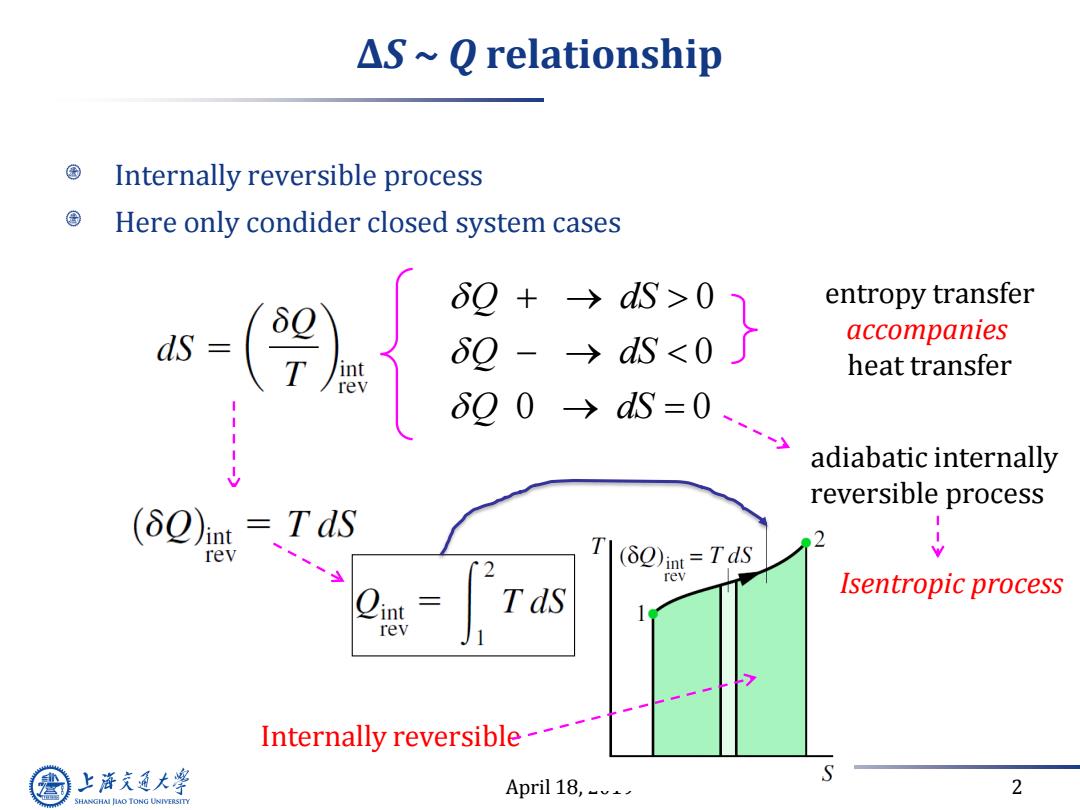
△S~Q relationship Internally reversible process Here only condider closed system cases 60 +→dS>0 entropy transfer s-9 8Q-→dS<0 accompanies heat transfer 00→s=0 adiabatic internally reversible process (δQ)mt=TdS rev 2 T(2)in=Tds rev Tds Isentropic process rev Internally reversible---- 上游充通大 April 18,_ 2 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2019 2 ∆S ~ Q relationship Internally reversible process Here only condider closed system cases 0 0 0 0 Q dS Q dS Q dS entropy transfer accompanies heat transfer adiabatic internally reversible process Isentropic process Internally reversible
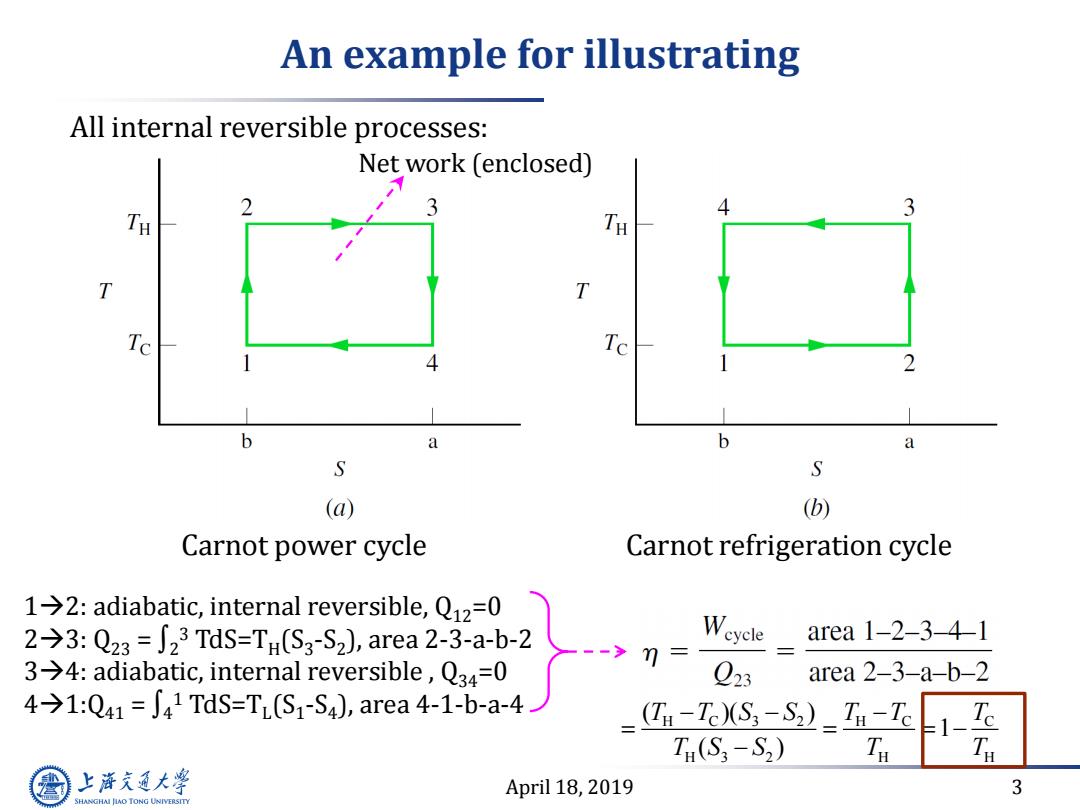
An example for illustrating All internal reversible processes: Net work (enclosed) TH TH Te 4 b a b a S S (a) (b) Carnot power cycle Carnot refrigeration cycle 1→2:adiabatic,internal reversible,Ql2=0 2→3:Q23=∫23TdS=TH(S3-S2),area2-3-a-b-2 Weyele area1-2-3-41 3→4:adiabatic,internal reversible,Q34=0 7= Q23 area 2-3-a-b-2 4→1:Q41=J41TdS=T(S1-S4),area4-1-b-a-4 (TH-Tc)(S3-S2)_TH-Tc 1-E T(S3-S2) T 上降文通大学 April 18,2019 3 SHANGHAI JLAO TONG UNIVERSITY
April 18, 2019 3 An example for illustrating Carnot refrigeration cycle 12: adiabatic, internal reversible, Q12=0 23: Q23 = ∫2 3 TdS=TH (S3 -S2 ), area 2-3-a-b-2 34: adiabatic, internal reversible , Q34=0 41:Q41 = ∫4 1 TdS=TL (S1 -S4 ), area 4-1-b-a-4 Carnot power cycle All internal reversible processes: Net work (enclosed) H C 3 2 H C C H 3 2 H H ( )( ) 1 ( ) T T S S T T T T S S T T
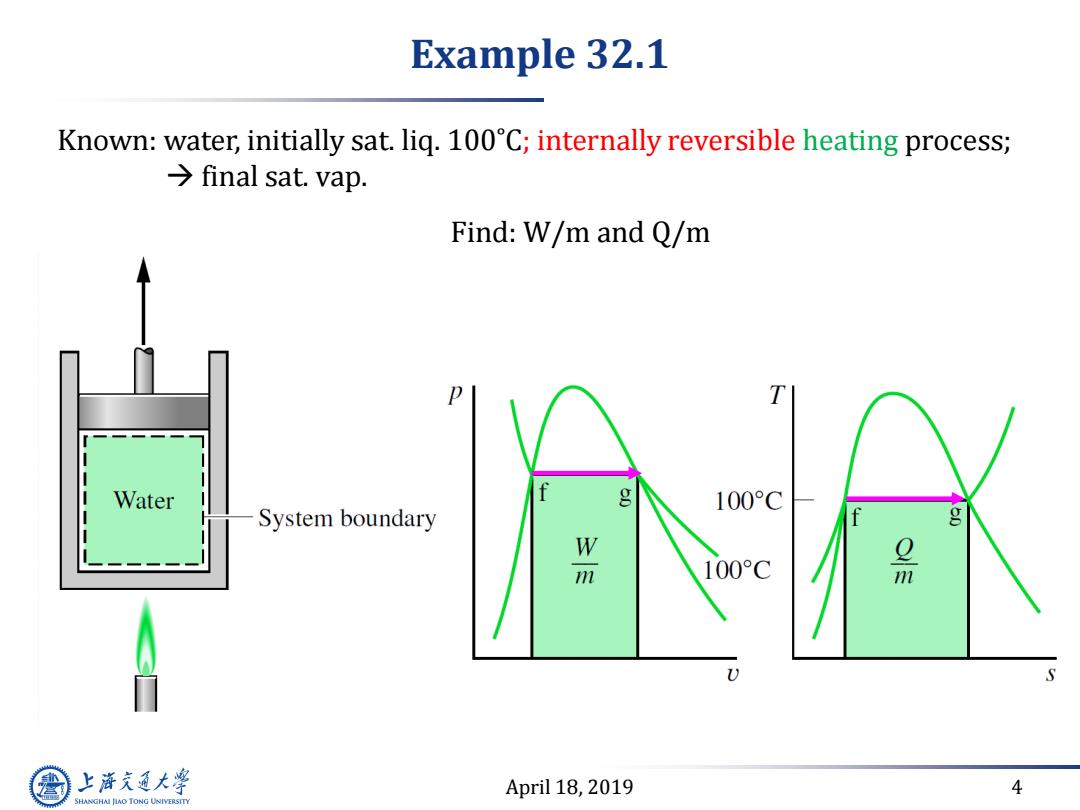
Example 32.1 Known:water,initially sat.liq.100C;internally reversible heating process; final sat.vap. Find:W/m and Q/m T Water g 100°C System boundary g 100°C 品 S 上游充通大 April 18,2019 4 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2019 4 Example 32.1 Known: water, initially sat. liq. 100˚C; internally reversible heating process; final sat. vap. Find: W/m and Q/m
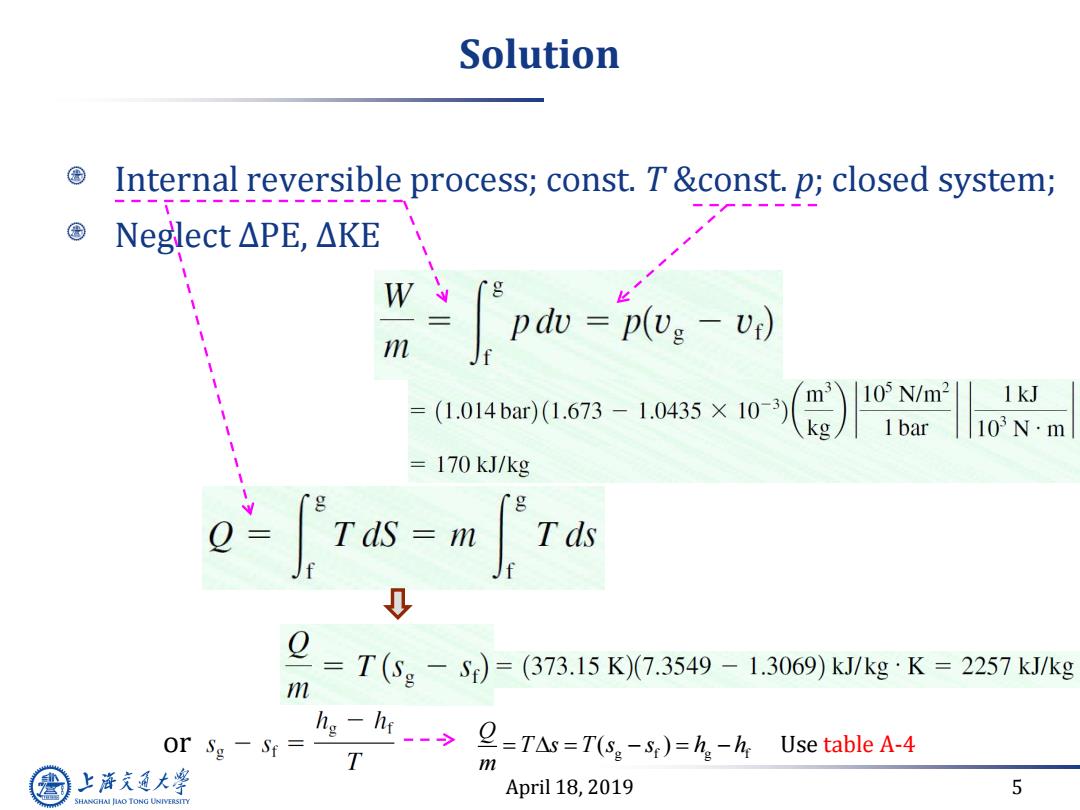
Solution Internal reversible process;const.T&const.p;closed system; Neglect△PE,△KE W m J pdo=plv:-v) (1.014bar)(1.673-1.0435×10-3) 1kJ = =170 kJ/kg Tas-m Tds m =T(Sg-S)=(373.15K)7.3549-1.3069)k/kg·K=2257kJkg or Sg-Sr= lisg-lt2TAs=T(ss)=h.-h Use tableA-4 T m 上游充通大学 April 18,2019 5 SHANGHAI JLAO TONG UNIVERSITY
April 18, 2019 5 Solution Internal reversible process; const. T &const. p; closed system; Neglect ∆PE, ∆KE or g f g f ( ) Q T s T s s h h m Use table A-4