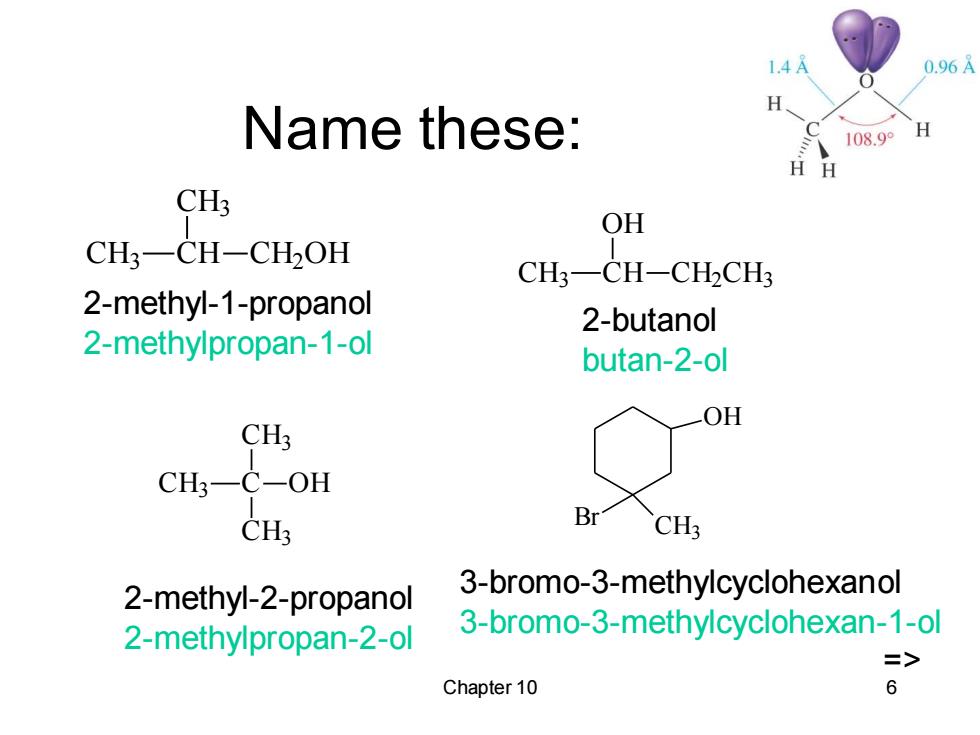
1.4 0.96A H Name these: 108.9° H CH3 OH CH3-CH-CH2OH CH3-CH-CH2CH3 2-methyl-1-propanol 2-butanol 2-methylpropan-1-ol butan-2-ol OH CH3 CH3一C-OH CH3 Br CH3 2-methyl-2-propanol 3-bromo-3-methylcyclohexanol 2-methylpropan-2-ol 3-bromo-3-methylcyclohexan-1-ol => Chapter 10 6
Chapter 10 6 Name these: CH3 CH CH3 CH2OH CH3 C CH3 CH3 OH CH3 CH OH CH2CH3 2-methyl-1-propanol 2-methylpropan-1-ol 2-methyl-2-propanol 2-methylpropan-2-ol 2-butanol butan-2-ol OH Br CH3 3-bromo-3-methylcyclohexanol 3-bromo-3-methylcyclohexan-1-ol =>
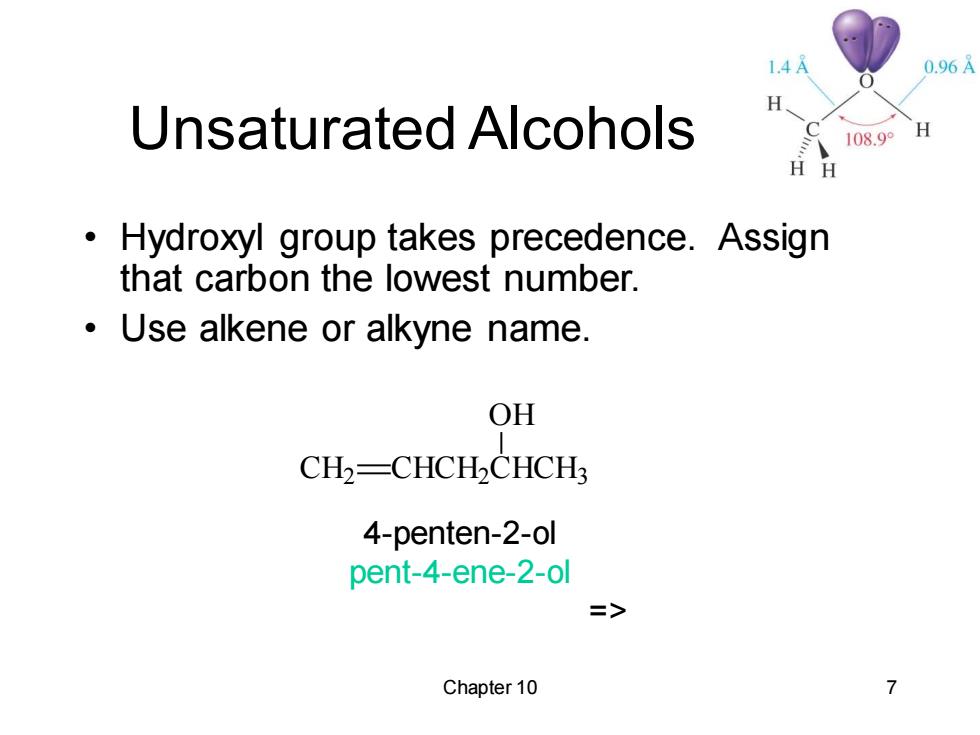
1.4A 0.96A H Unsaturated Alcohols 108.9° H H Hydroxyl group takes precedence.Assign that carbon the lowest number. Use alkene or alkyne name. OH CH2-CHCH-CHCH3 4-penten-2-ol pent-4-ene-2-ol => Chapter 10
Chapter 10 7 Unsaturated Alcohols • Hydroxyl group takes precedence. Assign that carbon the lowest number. • Use alkene or alkyne name. 4-penten-2-ol pent-4-ene-2-ol => CH2 CHCH2 CHCH3 OH

1.4A 0.96A Naming Priority 108.9° H H ·Acids ·Alkenes ·Esters ·Alkynes ·Aldehydes ·Alkanes ·Ketones ·Ethers ·Alcohols ·Halides ·Amines > Chapter 10 8
Chapter 10 8 Naming Priority • Acids • Esters • Aldehydes • Ketones • Alcohols • Amines • Alkenes • Alkynes • Alkanes • Ethers • Halides =>
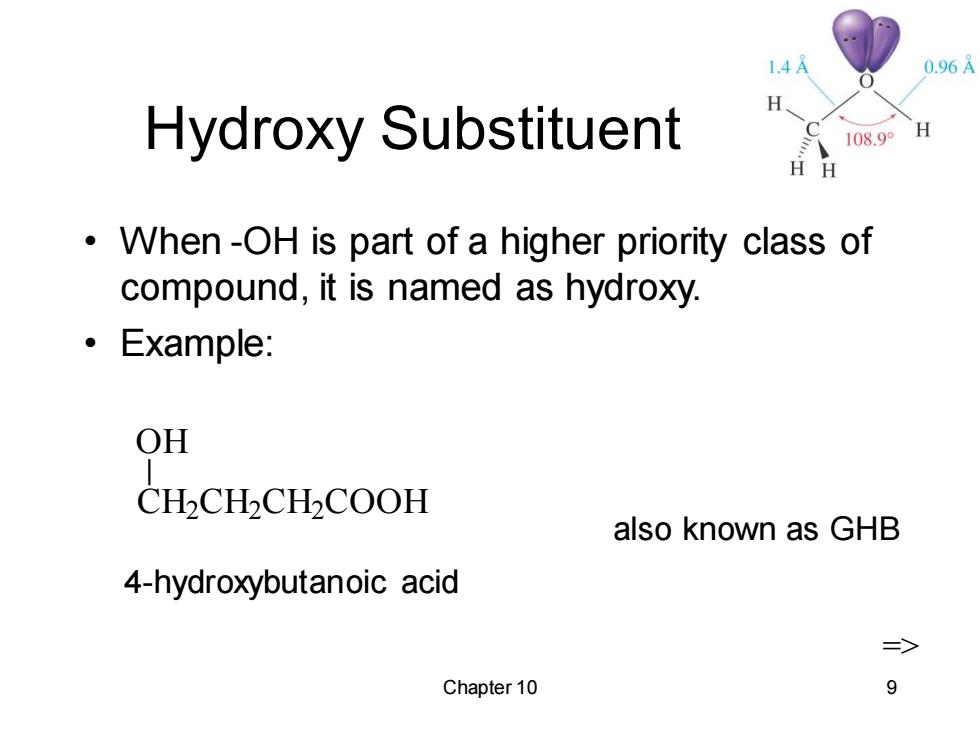
1.4A 0.96A Hydroxy Substituent 108.9° H H H When-OH is part of a higher priority class of compound,it is named as hydroxy. 。Example: OH CHCHCHCOOH also known as GHB 4-hydroxybutanoic acid => Chapter 10 9
Chapter 10 9 Hydroxy Substituent • When -OH is part of a higher priority class of compound, it is named as hydroxy. • Example: CH2 CH2 CH2 COOH OH 4-hydroxybutanoic acid also known as GHB =>

1.4A 0.96A Common Names 108.9° H H Alcohol can be named as alkyl alcohol. Useful only for small alkyl groups. 。Examples: CH3 OH CH3一CH-CH2OH CH3一CH-CH2CH3 isobutyl alcohol sec-butyl alcohol => Chapter 10 10
Chapter 10 10 Common Names • Alcohol can be named as alkyl alcohol. • Useful only for small alkyl groups. • Examples: CH3 CH CH3 CH2OH CH3 CH OH CH2CH3 isobutyl alcohol sec-butyl alcohol =>