
600 Experiments on the Diffraction of Cathode Rays. By G.P.THOMSON,M.A.,Fellow of Corpus Christi College and Professor of Natural Philosophy in the University of Aberdeen (Communicated by Sir Joseph Thomson,F.R.S.-Received November 4,1927.) [PLATH 19.] 1.M.L.de Broglie has introduced a theory of mechanics according to which a moving particle behaves as a group of waves whose velocity and wave-length are governed by the speed and mass of the particle.In fact if mo is the mass for slow speed and the speed of a freely moving particle,the wave-length is given by =hv1-2/c2/mov,and the wave velocity V by V=calv,the group velocity being v,the velocity of the particle.Here c is the velocity of light and it will be seen that the wave velocity is greater than c.There is nothing impossible in this because the waves are regarded as purely geometrical -"phase waves"-not as carrying energy.Compare,in ordinary optical theory,the case of substances,such as sodium,for which the refractive index is less than unity.The above is for free space;in the presence of a field of force V varies,and the consequent bending of the waves byrefraction corresponds, on the new theory,to the deviation of the path of the particle by the field of force,on the old. The consequences of thistheory have been workedout by de Broglie,Schrodinger and others and applied to problems in spectroscopy where they have provided the solution of several outstanding difficulties left by the older theory of orbits. In view,however,of the extremely fundamental nature of the theory it is highly desirable that it should rest on more direct evidence,and,in particular,that it should be shown capable of predicting as well as of merely explaining. Dymond*has obtained some remarkable results on the scattering of slow elec- trons in helium which are of the general nature to be expected in this theory, but our knowledge of the structure of helium,together with the mathematical difficulties of the problem have so far prevented any exact comparision of the theory with experiment.Davisson and Kunsmanf and Davisson and Germer have obtained results on the reflection of slow electrons from the surfaces of crystals,especially nickel,which show good qualitative agreement with the *‘Nature,.'vol.118,p.336(1926). ↑‘Phys.Rev.,'vol.22,p.243(1923). 年Nature,'voL.119,p.558(1927. The Royal Society is collaborating with JSTOR to digitize,preserve,and extend access to Proceedings of the Royal Society of London.Series A.Containing Papers of a Mathematical and Physical Character. www.jstor.org
The Royal Society is collaborating with JSTOR to digitize, preserve, and extend access to Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character. www.jstor.org ®

Eperiments on Diffraction of Cathode Rays. 60] theory but a discrepancy of 30 per cent.in certain magnitudes.It is hoped that the experiments described in this paper will advance the matter a stage further.They are a development of some experiments of which a preliminary account appeared recently in 'Nature.'* 2.These experiments were begun last year with the idea of extending Dymond's experiments on scattering to solid films and faster electrons,where it seemed probable that the technique developed for dealing with positive ray- scattering could be applied with advantage.The results now obtained,how- ever,are best looked at from a slightly different point of view to that applicable to Dymond's work,namely,as follows. On the de Broglie theory the electron is considered as a group of waves and its motion through matter is determined by considerations of scattering and diffraction.For electrons of 25,000 volts energy the wave-length a calculated from the above formula is about 0.75 x 10-9 cm.This is of the order of that of hard X-rays and the waves associated with electrons of this energy should behave in many respects like hard X-rays.In fact on this view there is a very close analogy between the two.The quantum effects of X-rays are regarded as due to centres of energy guided by waves,while in the case of the electrons the motion of the electric charge,in which the energy is centred,is regarded as taking place along the rays of the group of phase waves associated with it.In particular the electrons should show diffraction effects when passed through a crystal identical with those shown by X-rays of the same wave-length.It is, perhaps,hardly necessary to say that this does not mean that the two are indistinguishable.Unlike X-rays the electrons are deflected by electric and magnetic fields.They carry a charge,and for equal wave-lengths,have much less energy and less penetrating power. 3.In essence the experiments to be described consist in sending a beam of approximately homogeneous cathode rays through a very thin film at normal incidence and receiving them on a photographic plate some distance behind. If the film consists of a number of minute crystals arranged at random we should get a pattern identical with that obtained in a Hull-Debye-Scherrer apparatus with X-rays of the same wave-length.If certain directions of crystal predomi- nate the pattern will be modified in a calculable manner.It will be seen below that both these cases have been found.The only other necessary condition is that the film shall be so thin that the electron in its passage through it is only scattered once,otherwise the pattern will be hopelessly blurred by the super- position of the deflections and will appear on the photographic plates as a Thomson and Reid,'Nature,'vol.119,p.890 (1927)
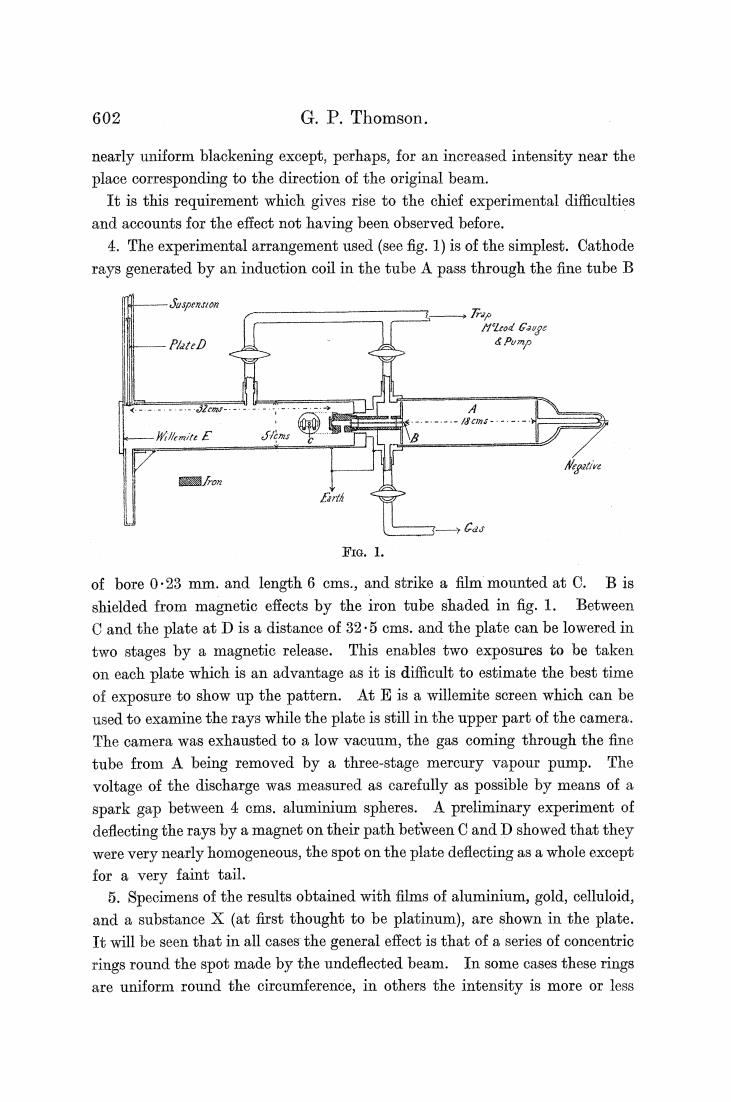
602 G.P.Thomson. nearly uniform blackening except,perhaps,for an increased intensity near the place corresponding to the direction of the original beam. It is this requirement which gives rise to the chief experimental difficulties and accounts for the effect not having been observed before. 4.The experimental arrangement used (see fig.1)is of the simplest.Cathode rays generated by an induction coil in the tube A pass through the fine tube B -Syspension .Trap MLod Gauge PlateD Pump 00 所肠两eE Eron Cas FIG.1. of bore 0.23 mm.and length 6 cms.,and strike a film mounted at C.B is shielded from magnetic effects by the iron tube shaded in fig.1.Between C and the plate at D is a distance of 32.5 cms.and the plate can be lowered in two stages by a magnetic release.This enables two exposures to be taken on each plate which is an advantage as it is difficult to estimate the best time of exposure to show up the pattern.At E is a willemite screen which can be used to examine the rays while the plate is still in the upper part of the camera. The camera was exhausted to a low vacuum,the gas coming through the fine tube from A being removed by a three-stage mercury vapour pump.The voltage of the discharge was measured as carefully as possible by means of a spark gap between 4 cms.aluminium spheres.A preliminary experiment of deflecting the rays by a magnet on their path between C and D showed that they were very nearly homogeneous,the spot on the plate deflecting as a whole except for a very faint tail. 5.Specimens of the results obtained with films of aluminium,gold,celluloid, and a substance X (at first thought to be platinum),are shown in the plate. It will be seen that in all cases the general effect is that of a series of concentric rings round the spot made by the undeflected beam.In some cases these rings are uniform round the circumference,in others the intensity is more or less
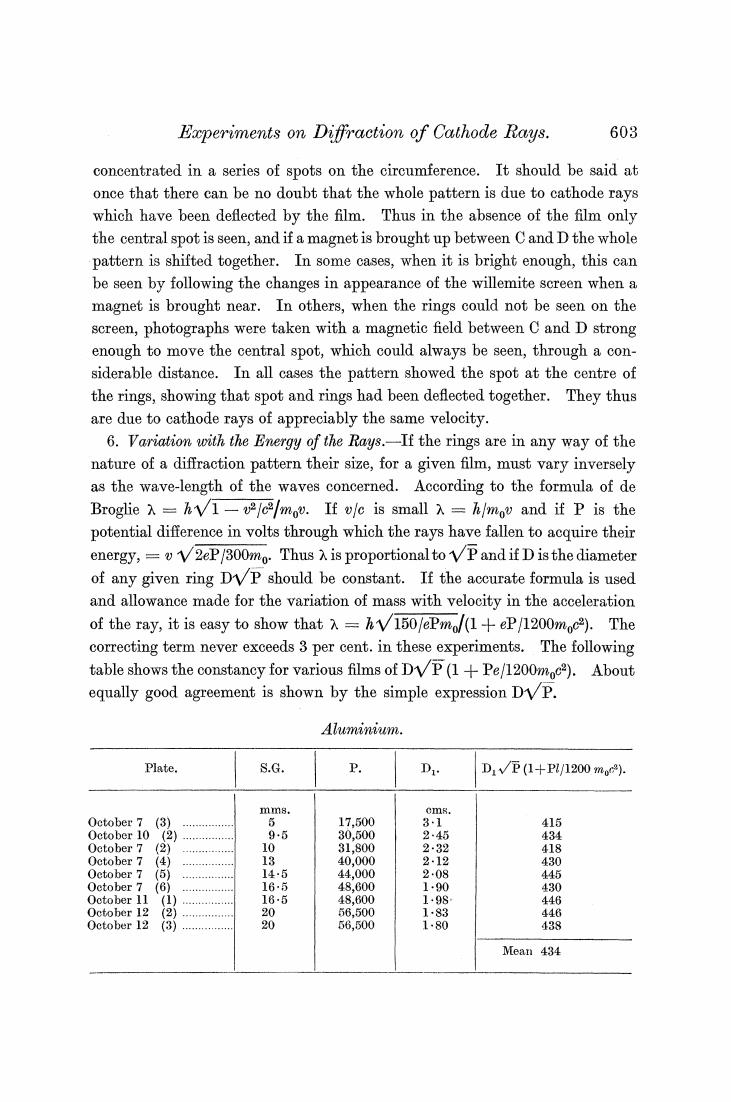
Eperiments on Diffraction of Cathode Rays. 603 concentrated in a series of spots on the circumference.It should be said at once that there can be no doubt that the whole pattern is due to cathode rays which have been deflected by the film.Thus in the absence of the film only the central spot is seen,and if a magnet is brought up between C and D the whole pattern is shifted together.In some cases,when it is bright enough,this can be seen by following the changes in appearance of the willemite screen when a magnet is brought near.In others,when the rings could not be seen on the screen,photographs were taken with a magnetic field between C and D strong enough to move the central spot,which could always be seen,through a con- siderable distance.In all cases the pattern showed the spot at the centre of the rings,showing that spot and rings had been deflected together.They thus are due to cathode rays of appreciably the same velocity. 6.Variation with the Energy of the Rays.-If the rings are in any way of the nature of a diffraction pattern their size,for a given film,must vary inversely as the wave-length of the waves concerned.According to the formula of de Broglie =hv1-v2/c/mov.If vfc is small =h/mov and if P is the potential difference in volts through which the rays have fallen to acquire their energy,=V2eP/300mo Thus is proportionaltoVPand if D isthe diameter of any given ring DVP should be constant.If the accurate formula is used and allowance made for the variation of mass with velocity in the acceleration of the ray,it is easy to show that=150/ePmo/(1+eP/1200moc2).The correcting term never exceeds 3 per cent.in these experiments.The following table shows the constancy for various films of DVP(1+Pe/1200mc). About equally good agreement is shown by the simple expression DP. Aluminium. Plate. 8.G. P. Dt D1√E(1+P/1200mo). October 7 (3 含 17.500 31 415 October 10 e 9-5 30,500 245 434 Oetober 7 10 31,800 232 418 Oetober 7 (4) 13 40,000 2-12 430 October 7 (5 -+k=t+=n44+ 145 44,000 2-08 445 October 7 165 48.600 1-90 430 October 11 (1) 16-5 48,600 1-98 446 October 12 e 20 66,500 1-83 446 October 12 年年年 2 56,500 1.80 438 Mean 434

604 G.P.Thomson. Gold. Plate. S.G. P D D'√P(1+Pe/100moc). mms. cms October 13 (1) …小… 75 24.600 2,50 398 Oetober 12 (4) 10 31,800 2·15 390 October 12 (5) 12.75 39,400 2-00 404 October 12 (6) 15·25 45,600 1·86 405 October 12 (7) 54,300 163 388 October 17 61,200 161 410 Mean 399 This close agreement is in itself strong evidence for the de Broglie theory, and justifies us in considering in detail the other consequences to be expected on this view. 7.Suppose that a beam of cathode rays is incident at an angle 0 on a plane of indices (k)of a small element of crystal.According to the Bragg formula it will be reflected provided that 2d sin 0=aA,where d is the spacing between parallel planes of the type ()If L is the distance of the plate,this will give rise to a mark on the plate at a distance D/2 from the central spot where D=4 0L=2n AL/d,assuming 0 is small. If a large number of small crystals are present arranged at random so that some are present at all angles we shall have a ring of diameter given by the above formula for every spacing d in the 02 crystal lattice and n=1,2,3,ete.This Primary Beam is,of course,the well-known Hull-Debye- F1G.2. Scherrer pattern for powdered crystals. If the crystals have definite orientations with respect to the film some of these rings will be absent.Thus consider a crystal with rectangular axes and suppose a (1,0,0)face is always (approximately)parallel to the film surface,we shall have reflections from (0,1,0)and (0,1,1)faces because the small angle 0 required can be provided by the slight divergence of the cathode ray beam,and by lack of flatness in the film.On the other hand the reflection from(1,1,1)will not appear because the rays would have to make a large angle with the plane of the film in order to be incident on (1,1,1)at the small angle required by the law 2 d sin 0=nA remembering that is of the order of 1/50 that of d.If,further,the crystals are so orientated that not merely is one axis perpendicular to the film,but the