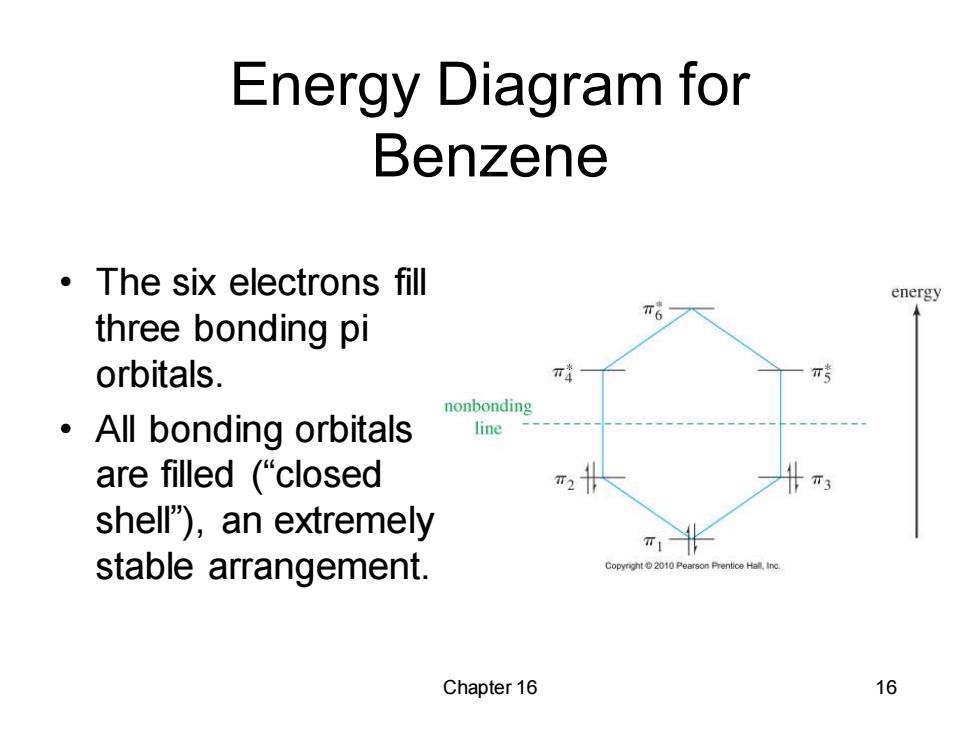
Energy Diagram for Benzene The six electrons fill energy three bonding pi orbitals. π 青 nonbonding ·All bonding orbitals line are filled(“closed shell"),an extremely TI- stable arrangement. Pearson Prentice ind Chapter 16 16
Chapter 16 16 Energy Diagram for Benzene • The six electrons fill three bonding pi orbitals. • All bonding orbitals are filled (“closed shell”), an extremely stable arrangement
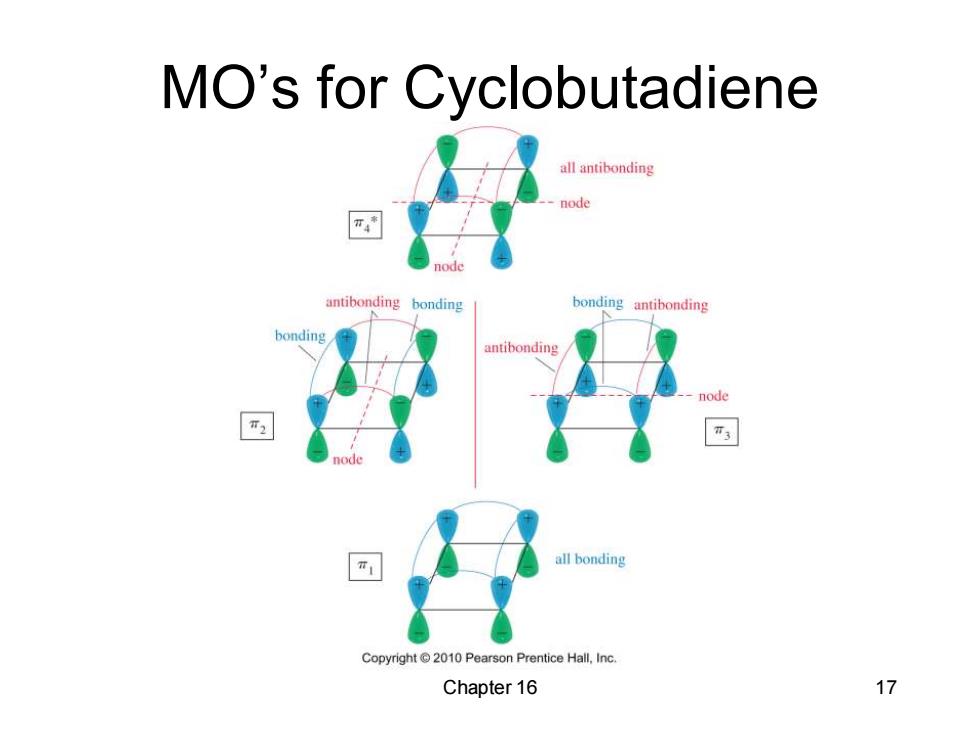
MO's for Cyclobutadiene all antibonding node antibonding bonding bonding antibonding bonding antibonding all bonding Copyright2010 Pearson Prentice Hall,Inc. Chapter 16 17
Chapter 16 17 MO’s for Cyclobutadiene
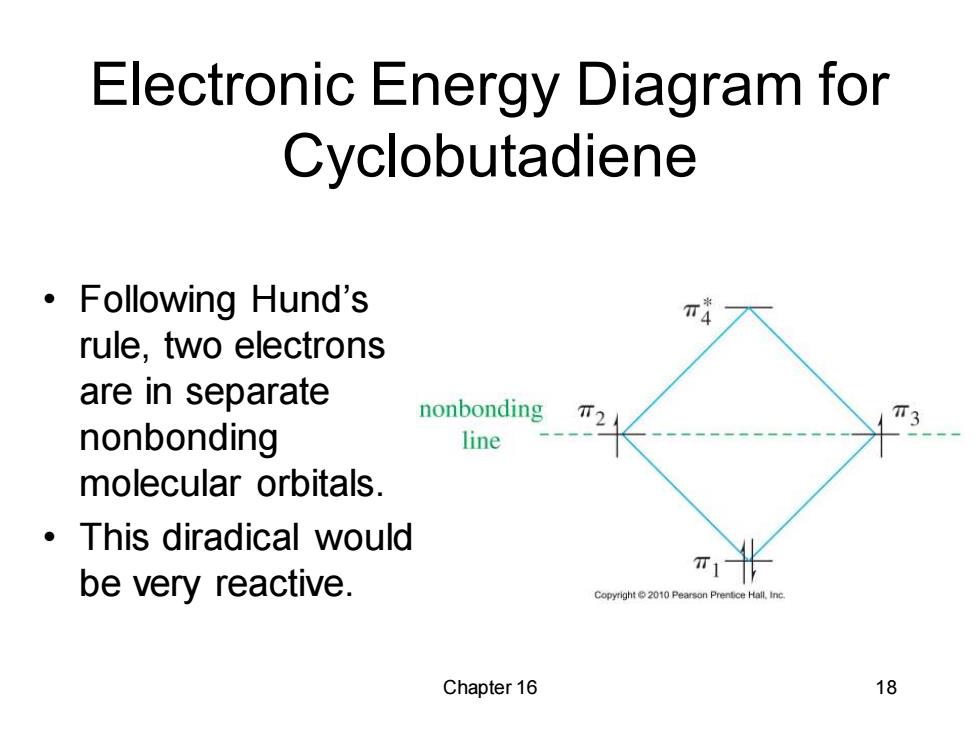
Electronic Energy Diagram for Cyclobutadiene ·Following Hund's π4 rule,two electrons are in separate nonbonding T2 nonbonding line molecular orbitals. ·This diradical would be very reactive. π1 Copyright 2010 Pearson Prentice Hall.Inc. Chapter 16 18
Chapter 16 18 Electronic Energy Diagram for Cyclobutadiene • Following Hund’s rule, two electrons are in separate nonbonding molecular orbitals. • This diradical would be very reactive
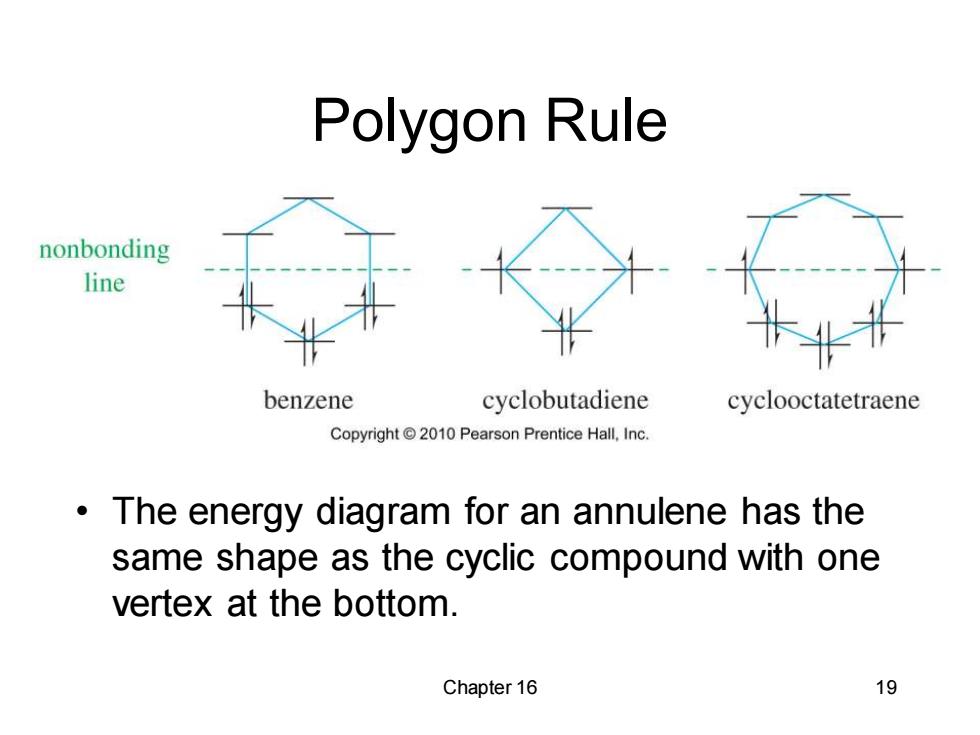
Polygon Rule nonbonding line benzene cyclobutadiene cyclooctatetraene Copyright2010 Pearson Prentice Hall,Inc. The energy diagram for an annulene has the same shape as the cyclic compound with one vertex at the bottom. Chapter 16 19
Chapter 16 19 Polygon Rule • The energy diagram for an annulene has the same shape as the cyclic compound with one vertex at the bottom

Aromatic Requirements Structure must be cyclic with conjugated pi bonds. Each atom in the ring must have an unhybridized p orbital(sp2 or sp). The p orbitals must overlap continuously around the ring.Structure must be planar (or close to planar for effective overlap to occur) Delocalization of the pi electrons over the ring must lower the electronic energy. Chapter 16 20
Chapter 16 20 Aromatic Requirements • Structure must be cyclic with conjugated pi bonds. • Each atom in the ring must have an unhybridized p orbital (sp2 or sp). • The p orbitals must overlap continuously around the ring. Structure must be planar (or close to planar for effective overlap to occur) • Delocalization of the pi electrons over the ring must lower the electronic energy