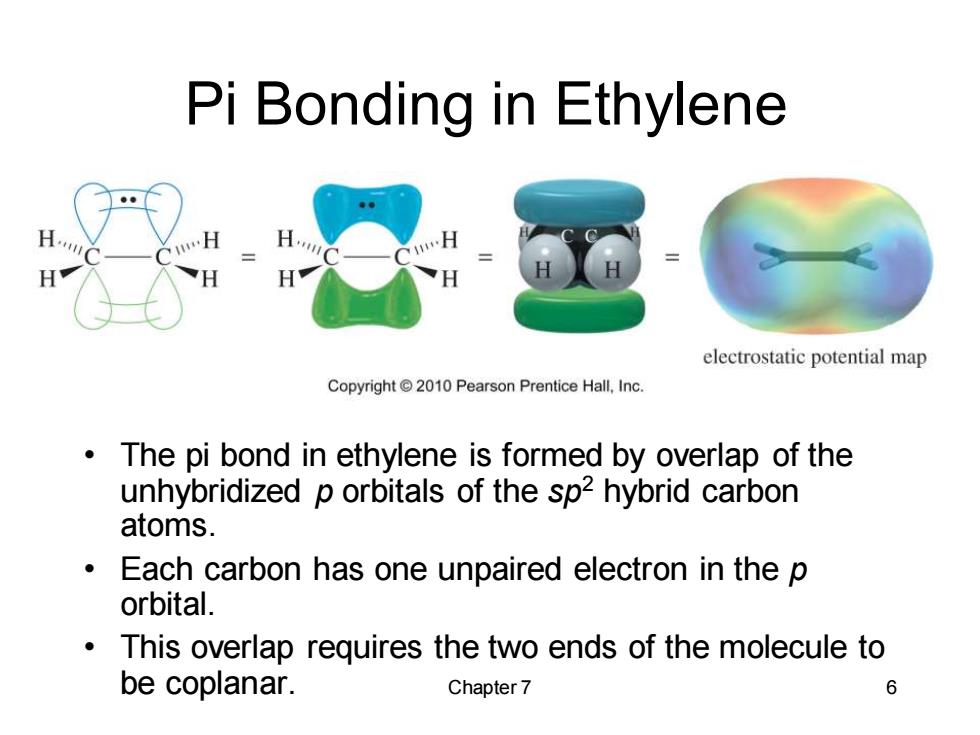
Pi Bonding in Ethylene electrostatic potential map Copyright 2010 Pearson Prentice Hall,Inc. The pi bond in ethylene is formed by overlap of the unhybridized p orbitals of the sp2 hybrid carbon atoms. Each carbon has one unpaired electron in the p orbital. This overlap requires the two ends of the molecule to be coplanar. Chapter 7 6
Chapter 7 6 Pi Bonding in Ethylene • The pi bond in ethylene is formed by overlap of the unhybridized p orbitals of the sp2 hybrid carbon atoms. • Each carbon has one unpaired electron in the p orbital. • This overlap requires the two ends of the molecule to be coplanar
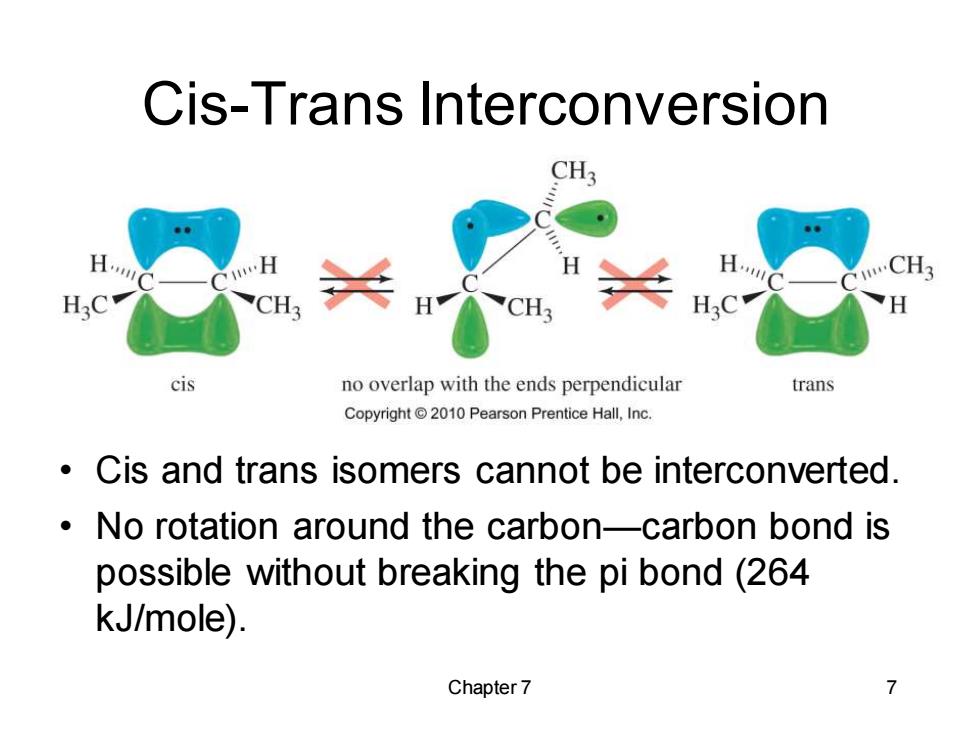
Cis-Trans Interconversion CH3 CH CH3 cis no overlap with the ends perpendicular trans Copyright2010 Pearson Prentice Hall,Inc. Cis and trans isomers cannot be interconverted. No rotation around the carbon-carbon bond is possible without breaking the pi bond(264 kJ/mole). Chapter 7 7
Chapter 7 7 Cis-Trans Interconversion • Cis and trans isomers cannot be interconverted. • No rotation around the carbon—carbon bond is possible without breaking the pi bond (264 kJ/mole)
Elements of Unsaturation Unsaturation:A structural element that decreases the number of hydrogens in the molecule by two. Double bonds and rings are elements of unsaturation. To calculate:Find number of hydrogens if they were saturated,subtract the actual number of hydrogens,then divide by 2. Chapter 7 8
Chapter 7 8 Elements of Unsaturation • Unsaturation: A structural element that decreases the number of hydrogens in the molecule by two. • Double bonds and rings are elements of unsaturation. • To calculate: Find number of hydrogens if they were saturated, subtract the actual number of hydrogens, then divide by 2
Example:Calculate the Unsaturations for a Compound with Formula C5Ha. First calculate the number of hydrogen atoms for a saturated compound with five carbons: (2×C)+2 (2X5)+2=12 Now subtract from this number the actual number of hydrogen atoms in the formula and divide by 2: 12-8=4=2 unsaturations 22 The compound has two unsaturations,they can be two double bonds,two rings,or one double bond and one ring. Chapter 7 9
Chapter 7 9 Example: Calculate the Unsaturations for a Compound with Formula C5H8 . • First calculate the number of hydrogen atoms for a saturated compound with five carbons: (2 x C) + 2 (2 x 5) + 2 = 12 • Now subtract from this number the actual number of hydrogen atoms in the formula and divide by 2: 12 – 8 = 4 = 2 unsaturations 2 2 • The compound has two unsaturations, they can be two double bonds, two rings, or one double bond and one ring

Elements of Unsaturation: Heteroatoms Halogens replace hydrogen atoms in hydrocarbons,so when calculating unsaturations,count halides as hydrogen atoms. Oxygen does not change the C:H ratio,so ignore oxygen in the formula. Nitrogen is trivalent,so it acts like half a carbon.Add the number of nitrogen atoms when calculating unsaturations. Chapter 7 10
Chapter 7 10 Elements of Unsaturation: Heteroatoms • Halogens replace hydrogen atoms in hydrocarbons, so when calculating unsaturations, count halides as hydrogen atoms. • Oxygen does not change the C:H ratio, so ignore oxygen in the formula. • Nitrogen is trivalent, so it acts like half a carbon. Add the number of nitrogen atoms when calculating unsaturations