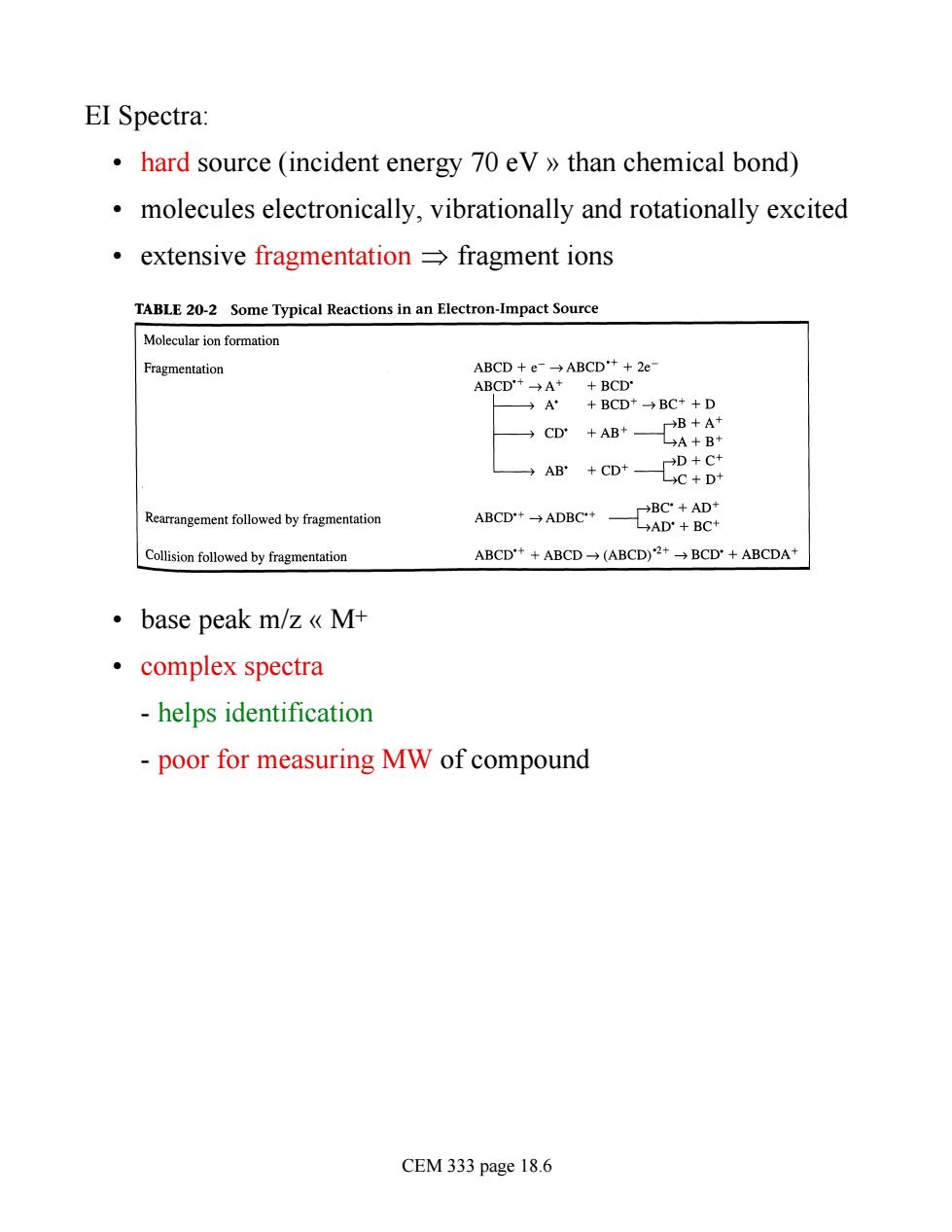
EI Spectra: hard source (incident energy 70 ev>>than chemical bond) molecules electronically,vibrationally and rotationally excited ·extensive fragmentation→fragment ions TABLE 20-2 Some Typical Reactions in an Electron-Impact Source Fragmentation ABCD+e→ABCD++2e ABCD+→A++BCD A+BCD*→BC++D →CD+AB*一CR+B AW +CD Rearrangement followed by fragmentation Colis followed by ABCD++ABCD→(ABCD)2+→BCD'+ABCDA+ ·base peak m/zMt ·complex spectra -helps identification poor for measuring MW of compound CEM 333 page 18.6
EI Spectra: • hard source (incident energy 70 eV » than chemical bond) • molecules electronically, vibrationally and rotationally excited • extensive fragmentation Þ fragment ions • base peak m/z « M+ • complex spectra - helps identification - poor for measuring MW of compound CEM 333 page 18.6
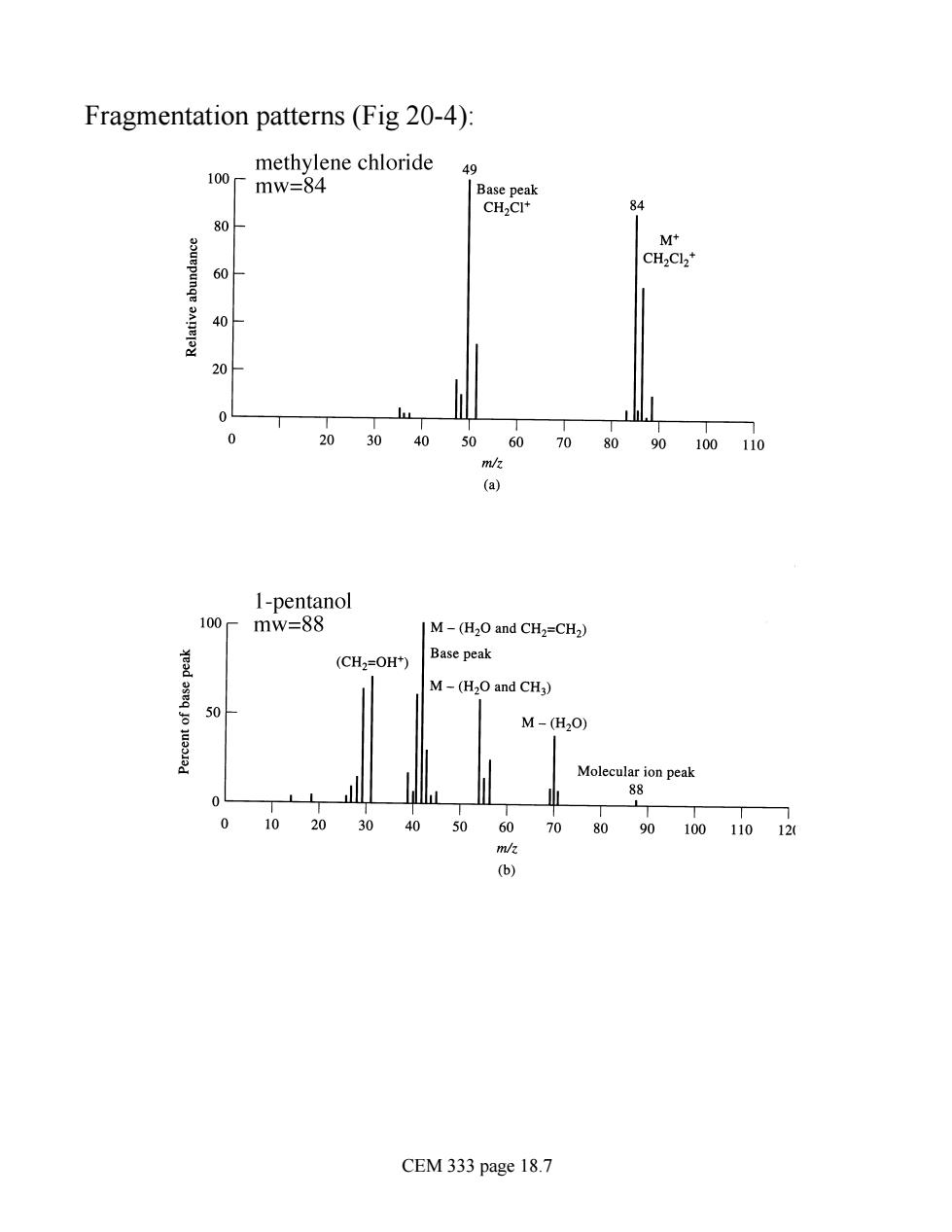
Fragmentation patterns(Fig 20-4) methylene chloride 100 49 mw=84 84 80人 60 40H 204 20 30 40 5060 90100110 (a) I-pentanol mw=88 |M-(H2O and CH2=CH2) (CH2=OH) Base peak M-(H2O and CH3) M-(H20) Molecula ion peak 40 50 60 80 9010011 (b) CEM 333 page 18.7
Fragmentation patterns (Fig 20-4): CEM 333 page 18.7
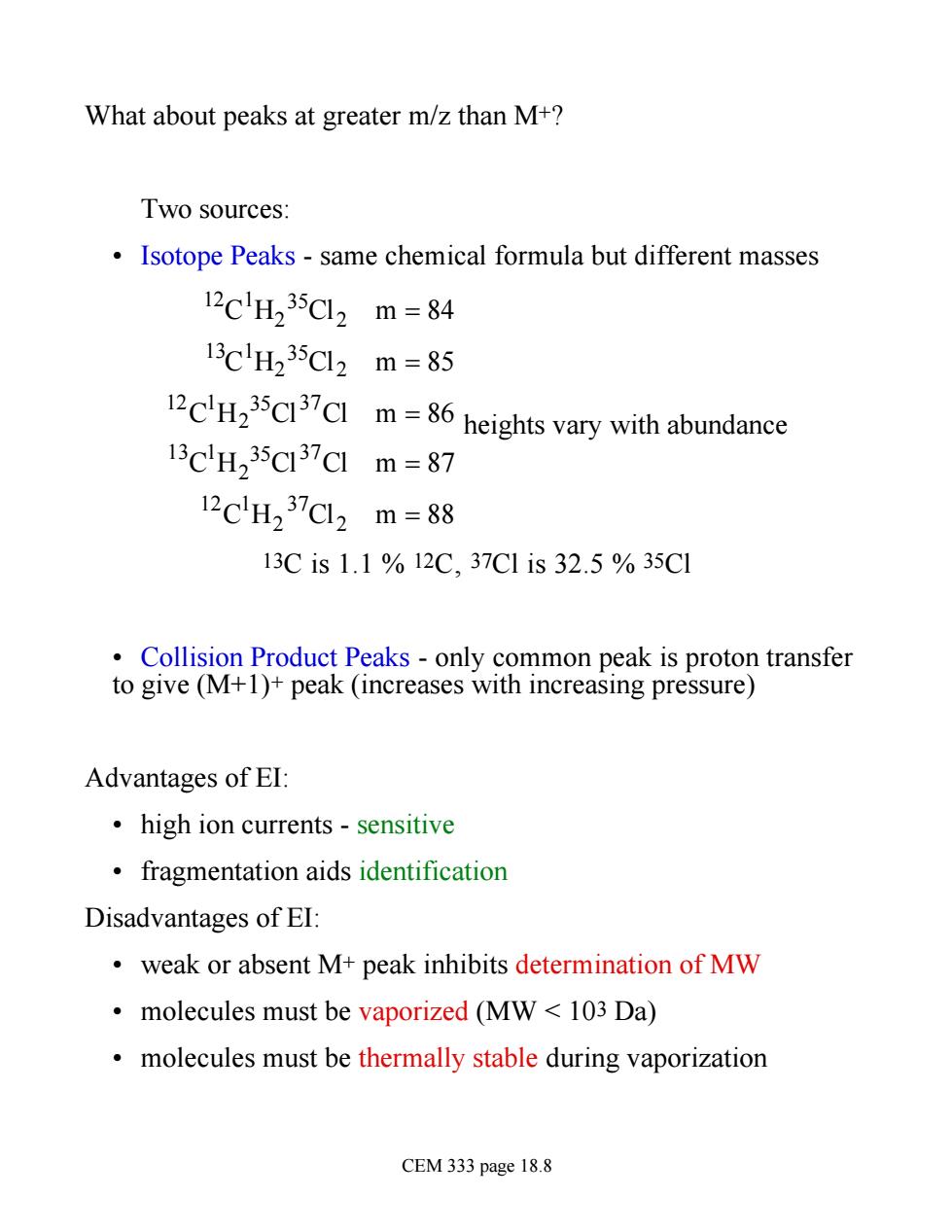
What about peaks at greater m/z than M+? Two sources: Isotope Peaks-same chemical formula but different masses 12clH235Cl2m=84 13clH235Cl2m=85 12CH235C13CIm heights vary with abundance 13CH235c37c1m=87 12CH237CL2m=88 13Cis1.1%12C,37C1is32.5%35C1 Collision Product Peaks -only common peak is proton transfer to give (M+1)+peak (increases with increasing pressure) Advantages of EI high ion currents-sensitive fragmentation aids identification Disadvantages of EI: weak or absent M+peak inhibits determination of MW molecules must be vaporized (MW 103 Da) molecules must be thermally stable during vaporization CEM 333 page 18.8
What about peaks at greater m/z than M+? Two sources: • Isotope Peaks - same chemical formula but different masses 12C 1H2 35Cl2 m = 84 13C 1H2 35Cl2 m = 85 12C 1H2 35Cl37Cl m = 86 13C 1H2 35Cl37Cl m = 87 12C 1H2 37Cl2 m = 88 heights vary with abundance 13C is 1.1 % 12C, 37Cl is 32.5 % 35Cl • Collision Product Peaks - only common peak is proton transfer to give (M+1)+ peak (increases with increasing pressure) Advantages of EI: • high ion currents - sensitive • fragmentation aids identification Disadvantages of EI: • weak or absent M+ peak inhibits determination of MW • molecules must be vaporized (MW < 103 Da) • molecules must be thermally stable during vaporization CEM 333 page 18.8