
SEC 2 Hybridization Molecular Shapes ■Hybridized orbitals are lower in energy because electron pairs are farther apart. Hybridization is LCAO Linus Pauling (1901-1994)was Within one atomjust the first person ever to receive tuo unshared Nobel prizes.He received the prior to bonding. 1954 Nobel prize in chemistry for his contributions to our understanding linear combination of atomic orbitals--- of chemical bonding.He received the LCAO 1962 Nobel peace prize for his efforts on behalf ofinternational control of nuclear weapons testing
SEC 2 Hybridization & Molecular Shapes ◼Hybridized orbitals are lower in energy because electron pairs are farther apart. ◼Hybridization is LCAO within one atom, just prior to bonding. linear combination of atomic orbitals--- LCAO

自秋标特材 Theories Valence Bond Theory价键理论 oconcerns itself with the formation of sigma and pi bonds. VSEPR:Valence Shell Electron Pair Repulsion Theory The premise of VSEPR is that the valence electron pairs surrounding an atom mutually repel each other,and will therefore adopt an arrangement that minimizes this repulsion,thus determining the molecular geometry. VSEPR addresses molecular shape through orbitals that are energetically accessible for bonding
Theories ◼Valence Bond Theory价键理论 ⚫concerns itself with the formation of sigma and pi bonds. ◼VSEPR: Valence Shell Electron Pair Repulsion Theory ⚫The premise of VSEPR is that the valence electron pairs surrounding an atom mutually repel each other, and will therefore adopt an arrangement that minimizes this repulsion, thus determining the molecular geometry. ⚫VSEPR addresses molecular shape through orbitals that are energetically accessible for bonding
自秋镜试对 Theories Molecular Orbital Theory ● model for understanding how atoms and electrons are assembled into molecules and polyatomic ions. ■Hybridization Theory杂化轨道理论 ● a covalent bond is formed by the overlap of two singly occupied hybrid or atomic orbitals. Hybrid atomic orbitals are created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals
Theories ◼Molecular Orbital Theory ⚫ model for understanding how atoms and electrons are assembled into molecules and polyatomic ions. ◼Hybridization Theory杂化轨道理论 ⚫ a covalent bond is formed by the overlap of two singly occupied hybrid or atomic orbitals. ⚫ Hybrid atomic orbitals are created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals
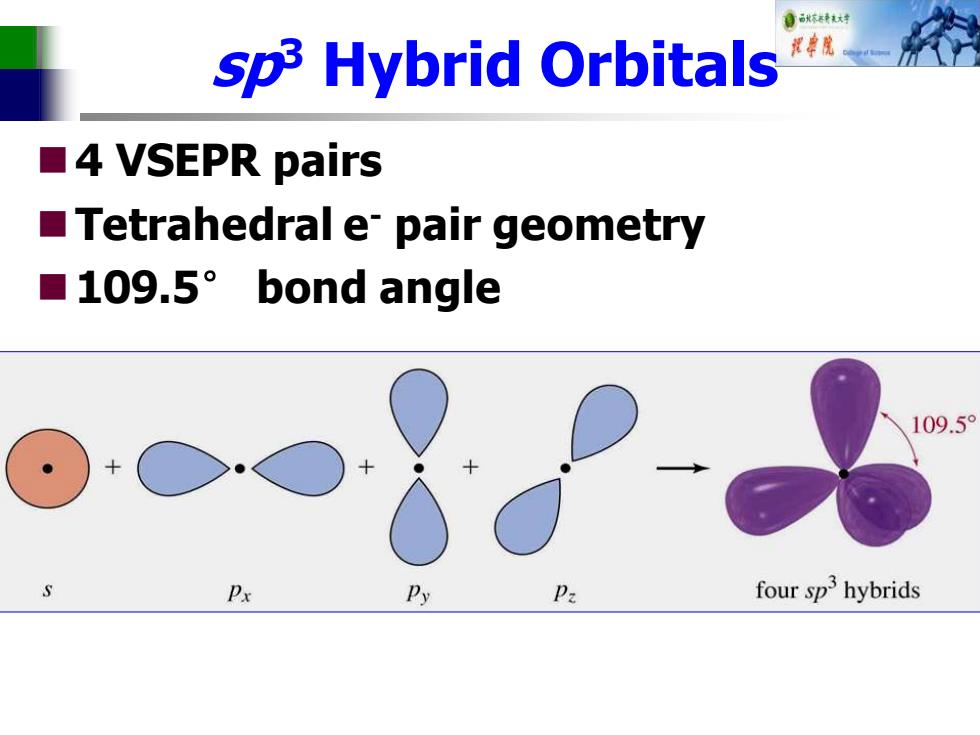
自标转对 sp3 Hybrid Orbitals ■4 VSEPR pairs Tetrahedral e pair geometry ■109.5°bond angle 109.5° Px Py Pa four sp3 hybrids
sp3 Hybrid Orbitals ◼4 VSEPR pairs ◼Tetrahedral e- pair geometry ◼109.5° bond angle

Computed sp3 Hybrid Orbitals Cartoon 109.5° (a)An sp3 orbital (b)Four sp3orbitals in a (c) tetrahedral arrangement
Computed sp3 Hybrid Orbitals