Proteolytic activation Many proteins/enzymes are made as inactive precursors that are activated by proteolytic cleavage:after this cleavage the enzymes fold into their catalytically active conformation. Inactive precursors are called proenzymes or zymogens. Proteolytic activation is irreversible -a different mechanism is required for inactivation of the enzyme. Examples:blood clotting,digestive proteases,peptide hormones (insulin),proteins involved in developmental processes (collagen),apoptosis (programmed cell death; caspases)and virus proteases (cuts multidomain viral proteins into their active forms,see 9.1.7)
Proteolytic activation • Many proteins/enzymes are made as inactive precursors that are activated by proteolytic cleavage: after this cleavage the enzymes fold into their catalytically active conformation. • Inactive precursors are called proenzymes or zymogens. • Proteolytic activation is irreversible -> a different mechanism is required for inactivation of the enzyme. • Examples: blood clotting, digestive proteases, peptide hormones (insulin), proteins involved in developmental processes (collagen), apoptosis (programmed cell death; caspases) and virus proteases (cuts multidomain viral proteins into their active forms, see 9.1.7)
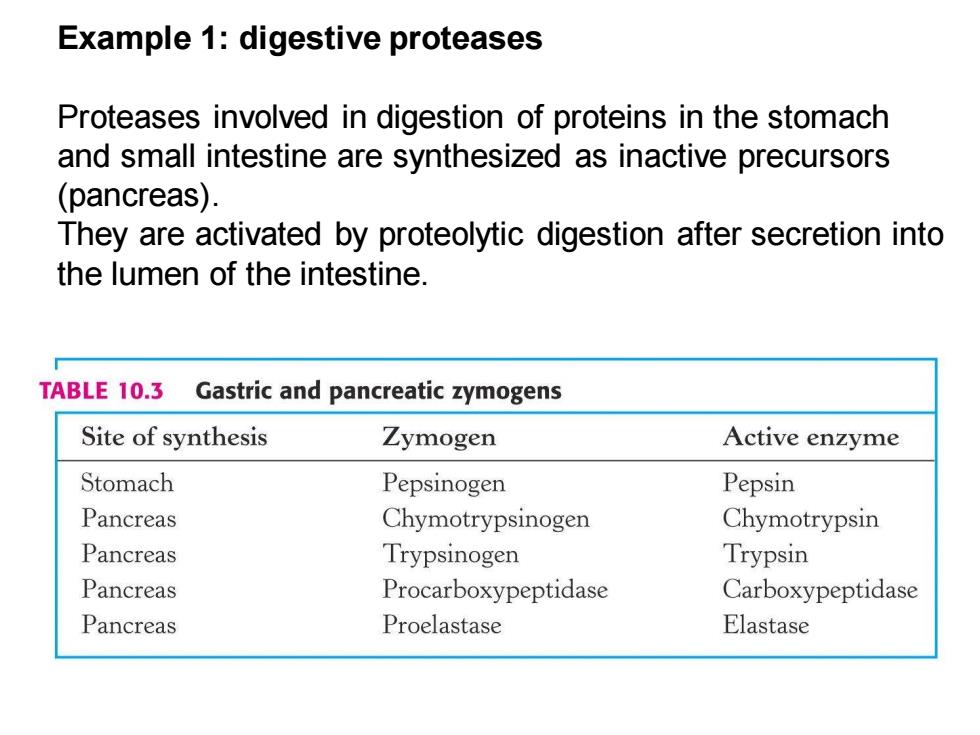
Example 1:digestive proteases Proteases involved in digestion of proteins in the stomach and small intestine are synthesized as inactive precursors (pancreas). They are activated by proteolytic digestion after secretion into the lumen of the intestine. TABLE 10.3 Gastric and pancreatic zymogens Site of synthesis Zymogen Active enzyme Stomach Pepsinogen Pepsin Pancreas Chymotrypsinogen Chymotrypsin Pancreas Trypsinogen Trypsin Pancreas Procarboxypeptidase Carboxypeptidase Pancreas Proelastase Elastase
Example 1: digestive proteases Proteases involved in digestion of proteins in the stomach and small intestine are synthesized as inactive precursors (pancreas). They are activated by proteolytic digestion after secretion into the lumen of the intestine
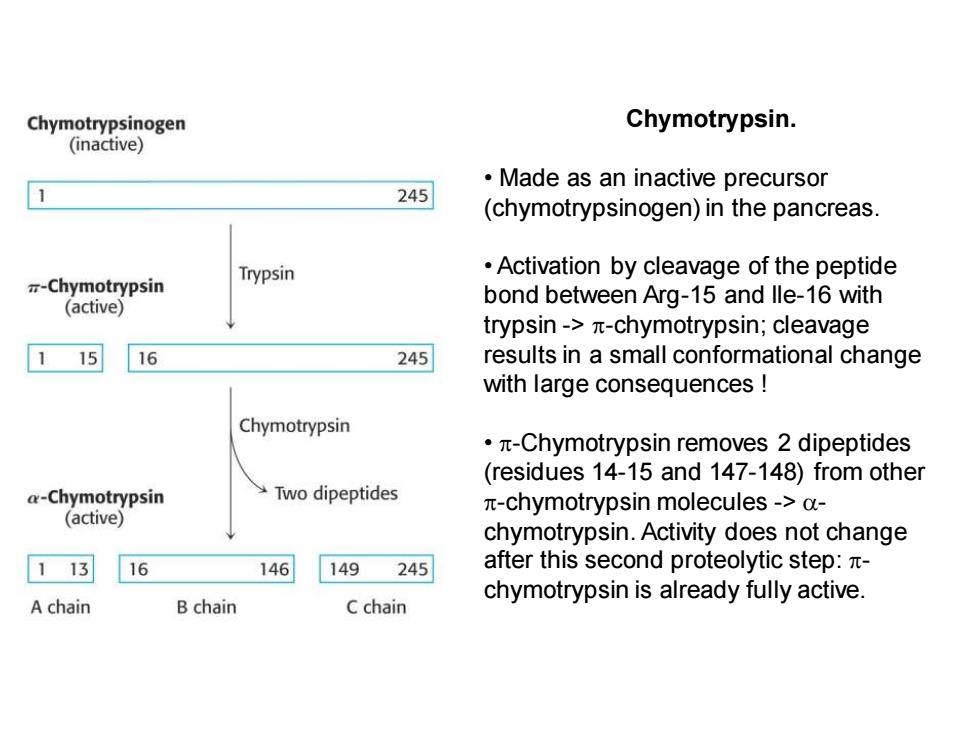
Chymotrypsinogen Chymotrypsin. (inactive) Made as an inactive precursor 245 (chymotrypsinogen)in the pancreas. Trypsin Activation by cleavage of the peptide -Chymotrypsin (active) bond between Arg-15 and lle-16 with trypsin->n-chymotrypsin;cleavage 15 16 245 results in a small conformational change with large consequences Chymotrypsin ·π-Chymotrypsin removes2 dipeptides (residues 14-15 and 147-148)from other a-Chymotrypsin Two dipeptides n-chymotrypsin molecules->a- (active) chymotrypsin.Activity does not change 1 13 16 146 149 245 after this second proteolytic step: chymotrypsin is already fully active. A chain B chain C chain
Chymotrypsin. • Made as an inactive precursor (chymotrypsinogen) in the pancreas. • Activation by cleavage of the peptide bond between Arg-15 and Ile-16 with trypsin -> -chymotrypsin; cleavage results in a small conformational change with large consequences ! • -Chymotrypsin removes 2 dipeptides (residues 14-15 and 147-148) from other -chymotrypsin molecules -> - chymotrypsin. Activity does not change after this second proteolytic step: - chymotrypsin is already fully active
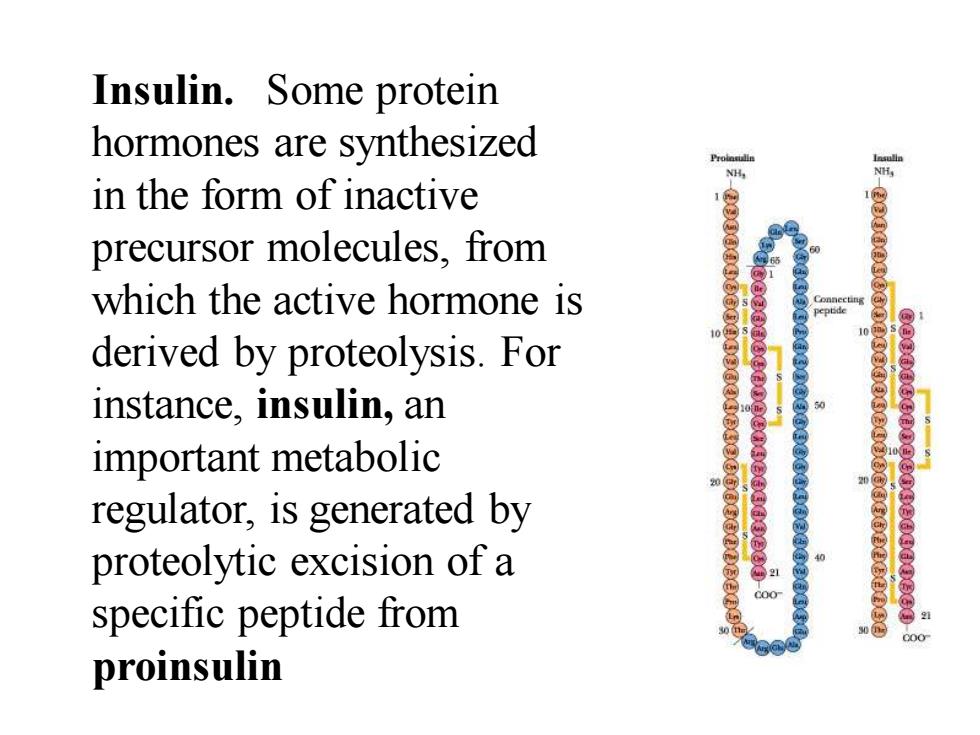
Insulin.Some protein hormones are synthesized in the form of inactive precursor molecules,from which the active hormone is y derived by proteolysis.For instance,insulin,an important metabolic regulator,is generated by proteolytic excision of a c00 specific peptide from proinsulin
Insulin. Some protein hormones are synthesized in the form of inactive precursor molecules, from which the active hormone is derived by proteolysis. For instance, insulin, an important metabolic regulator, is generated by proteolytic excision of a specific peptide from proinsulin
Iso☑ymes (A) LDH (lactate LDH-1 田 dehydrogenase) LDH-2 isozymes differ in their LDH-3 9 8 8 allosteric regulation by LDH-4 8 8 LDH-5 pyruvate. 9 -5 -1 +12 +21 Adult (B) Red Heart Kidney blood cell Brain Leukocyte Muscle Liver Ma HMs H2M2 HgM Ha
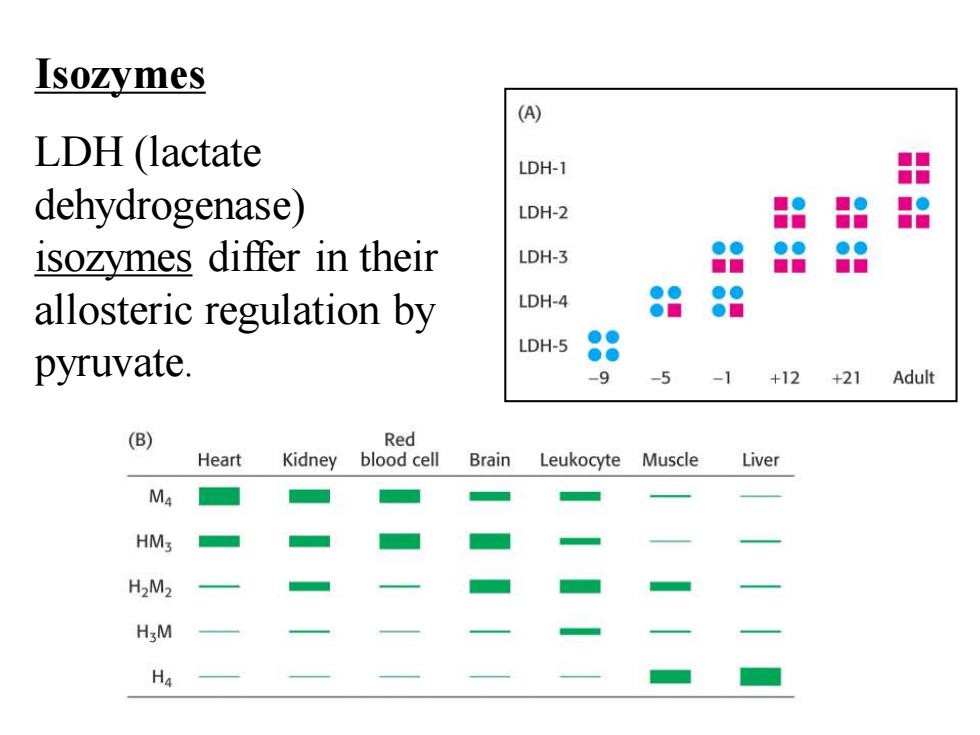
Isozymes LDH (lactate dehydrogenase) isozymes differ in their allosteric regulation by pyruvate