
Efficiency of an Engine If O is the heat taken from the hot reservoir and 22 is the heat dumped to the cold reservoir,then according to the 1st Law, 1211-121 lwl Now,we would like to reduce 22,the heat dumped,to a minimum. I22=I2l-w例 We want the efficiency, nc wi {net work done)/heat taken} 16 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
16 Efficiency of an Engine If Q1 is the heat taken from the hot reservoir and Q2 is the heat dumped to the cold reservoir, then according to the 1st Law, |Q1 | - |Q2 | = |w| Now, we would like to reduce Q2 , the heat dumped, to a minimum. |Q2 | = |Q1 | - |w| We want the efficiency, hC = |w|/|Q1 | = {net work done}/{heat taken} PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
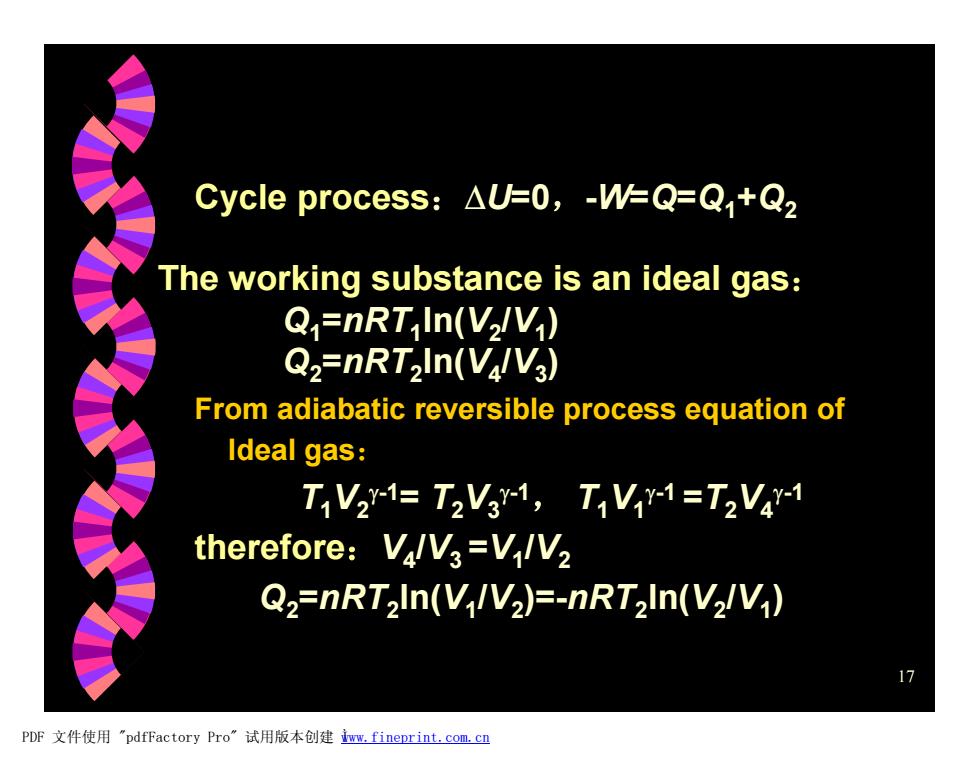
Cycle process:AU=0,-W=Q=Q+Q2 The working substance is an ideal gas: Q=nRT In(V/V) Q2=nRT2In(V/V3) From adiabatic reversible process equation of Ideal gas: T1V21=T2V31,T1Vr-1=T2V41 therefore:VlV3=V/V2 Q2=nRT2In(V/V2)=-nRT2In(V2/V) 17 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
17 From adiabatic reversible process equation of Ideal gas: T1V2 g-1= T2V3 g-1 , T1V1 g-1 =T2V4 g-1 therefore:V4 /V3 =V1 /V2 Q2=nRT2 ln(V1 /V2 )=-nRT2 ln(V2 /V1 ) Cycle process:DU=0,-W=Q=Q1+Q2 The working substance is an ideal gas: Q1=nRT1 ln(V2 /V1 ) Q2=nRT2 ln(V4 /V3 ) PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

7= W=2+Q2 0, 0 nRT In V2- nRT2 In V V V-T-T nRT In T V 9+02--T, 2+ 0, T 92=0 Conclusion: .For a cyclic reversible process,the sum of Q/T is equal to zero. .Efficiency of Carnot Engine,nc only depends on the hot reservoir T and the cold reservoir T2,is not related to the working matter. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint..com,c四
18 1 1 2 1 2 1 1 2 2 1 2 1 1 1 2 1 ln ln ln T T T V V nRT V V nRT V V nRT Q Q Q Q W - = - = + h = - = 1 1 2 1 1 2 T T T Q Q Q - = + 0 2 2 1 1 + = T Q T Q Conclusion: •For a cyclic reversible process,the sum of Qr /T is equal to zero . •Efficiency of Carnot Engine, hC only depends on the hot reservoir T1 and the cold reservoir T2 , is not related to the working matter. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

The Second Law of thermodynamics State the Second Law of thermodynamics: Clausius It is impossible that the cold body transfers heat to the hot. Kelvin No process is possible in which the sole result is the absorption of heat from a reservoir and its conversion into work. Entropy increasing principle The entropy of an isolated system increases during any natural process. PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
19 TTheheSSeeccoonndd L Laawwoof f the therrmmododynynaammicsics No process is possible in which the sole result is the absorption of heat from a reservoir and its conversion into work. No process is possible in which the sole result is the absorption of heat from a reservoir and its conversion into work. Clausius Kelvin It is impossible that the cold body transfers heat to the hot. It is impossible that the cold body transfers heat to the hot. SState the Second Law of thermodynamics: tate the Second Law of thermodynamics: The entropy of an isolated system increases during any natural process. Entropy increasing principle PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Carnot Principle The efficiency of the reversible engine is the greatest among all the engines working between the same hot reservoir and cold reservoir no n Deduction All reversible engines have the same efficiency regardless of their construction nc=n Conclusion T-1≥ Q1+Q2 0+ 02≤0 Irreversible T 0, T T2 Reversible 20 PDF文件使用"pdfFactory Pro”试用版本创建iw,fineprint.com,cm
20 CCaar rnonot tPPr ri inncci ipplele The efficiency of the reversible engine is the greatest among all the engines working between the same hot reservoir and cold reservoir hc > h ¢ DDeeduductcti ionon All reversible engines have the same efficiency regardless of their construction hc = h ¢ 1 1 2 1 1 2 Q Q Q T T T + ³ - 0 2 2 1 1 + £ T Q T Q Conclusion Irreversible Reversible PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn Ì