The thermodynaminixtures PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint..com,c四
1 The thermodynamics of mixtures PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Introduction Chemistry deals with mixtures,including mixtures of substances that can react together. Therefore,we need to generalize the concepts introduced so far to deal with substances that are mixed together. As a first step towards dealing with chemical reactions, here we consider mixtures of substances that do not react together.At this stage we deal mainly with binary mixtures (mixtures of two components,A and B). Another restriction of this chapter is that we consider mainly non-electrolyte solutions,in which the solute is not present as ions PDF文件使用"pdfFactory Pro”试用版本创建織ifineprint..com,cn
2 Introduction Chemistry deals with mixtures, including mixtures of substances that can react together. Therefore , we need to generalize the concepts introduced so far to deal with substances that are mixed together. As a first step towards dealing with chemical reactions, here we consider mixtures of substances that do not react together. At this stage we deal mainly with binary mixtures (mixtures of two components, A and B). Another restriction of this chapter is that we consider mainly non-electrolyte solutions, in which the solute is not present as ions . PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿ駌ÿ www.fineprint.com.cn

The solute dissolves in the solveni The solubility of a substance is the maximum amount that can dissolve in a fixed amount of a particular solvent. When the components of a mixture are homogeneous/y intermingled it is called a solution. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,c四
3 n The solute dissolves in the solvent. n The solubility of a substance is the maximum amount that can dissolve in a fixed amount of a particular solvent. n When the components of a mixture are homogeneously intermingled it is called a solution. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿÿwww.fineprint.com.cn
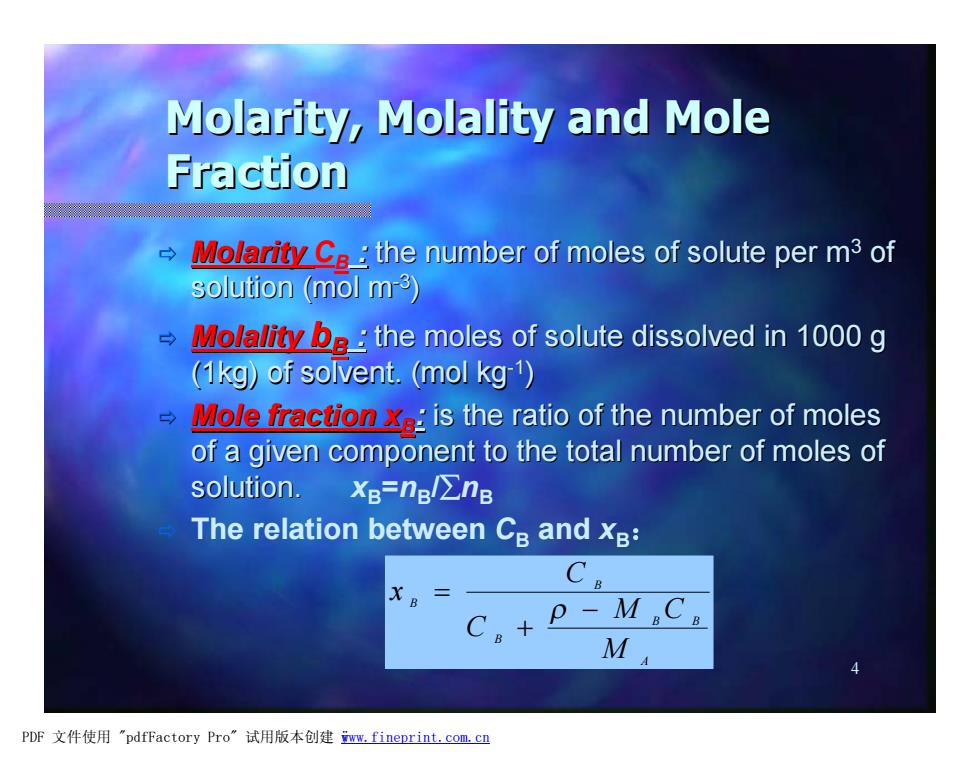
Molarity,Molality and Mole Fraction → Molarity Ca the number of moles of solute per m3 of solution (mol m3) Molality o the moles of solute dissolved in 1000 g (1kg)of solvent.(mol kg-1) Mole fraction is the ratio of the number of moles of a given component to the total number of moles of solution. XB=nBl∑nB The relation between Cs and xB: C> C。+ P-MC M方 PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,c四
4 Molarity, Molality and Mole Fraction ð MolarityCB : the number of moles of solute per m3 of solution (mol m-3 ) ð Molality bB : the moles of solute dissolved in 1000 g (1kg) of solvent. (mol kg-1 ) ð Mole fraction xB: is the ratio of the number of moles of a given component to the total number of moles of solution. xB=nB /ånB ð The relation between CB and xB: A B B B B B M M C C C x - + = r PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn

The relation between bB and xB: b B +b M PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
5 n The relation between bB and xB: B A B B b M b x + = 1 PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn