
Synnove Liaaen-Jensen configuration,and also EZ configuration of double bonds.In recent years,there has been significant in develo application is outlined briefly in Chapter 3. Recent examples where modern NMR techniques have been used to establish omplicated carotenoid structures,including relative stereochemistry,can be found in the Carotenoids Handbook.e.g.the Cs norcarotenoids peridinin (558)and pyrrhoxanthin (556),the lactoside P457(280.1).isolated as the 13-cis(13E)isomer,the 3,6:3'6'-diepoxide eycloviolaxanthin (259.3)and the allenic murillaxanthin (421.1)as well as carotenoids carbon skeletal substituents,such as pittosporumxanthin A (259.1),botryoxanthin A(283.1)and the braunixanthins(294.1). (558)peridinin s)pymhoxan 280.1)P 安
10 Synnøve Liaaen-Jensen OOCCH3 O O O OH OH O O OH configuration, and also E/Z configuration of double bonds. In recent years, there has been significant progress in developing new 2-dimensional (2D) NMR techniques [2], in particular COSY, ROESY, HSQC and HMBC, which are now used routinely. Their application is outlined briefly in Chapter 3. Recent examples where modern NMR techniques have been used to establish complicated carotenoid structures, including relative stereochemistry, can be found in the Carotenoids Handbook, e.g. the C37 norcarotenoids peridinin (558) and pyrrhoxanthin (556), the lactoside P457 (280.1), isolated as the 13-cis (13E) isomer, the 3,6:3’6’-diepoxide cycloviolaxanthin (259.3) and the allenic murillaxanthin (421.1) as well as carotenoids with peculiar and complex carbon skeletal substituents, such as pittosporumxanthin A (259.1), botryoxanthin A (283.1) and the braunixanthins (294.1). HO OCOCH3 O O O OH . (558) peridinin (556) pyrrhoxanthin HO OH O O10H21C12O CHO . (280.1) ‘P457’ (259.3) cycloviolaxanthin

Structure and Chirality (421.1)murillaxanthin 83.1)botryoxanthin (294.1)braunixanthins
Structure and Chirality 11 O O O HO OH O H H O O O O O OH OMe MeO O OH ( )x braunixanthin 1: x = 8 braunixanthin 2: x = 9 CH2OCOCH3 O O HO OH OH . (421.1) murillaxanthin (259.1) pittosporumxanthin A (283.1) botryoxanthin A (294.1) braunixanthins
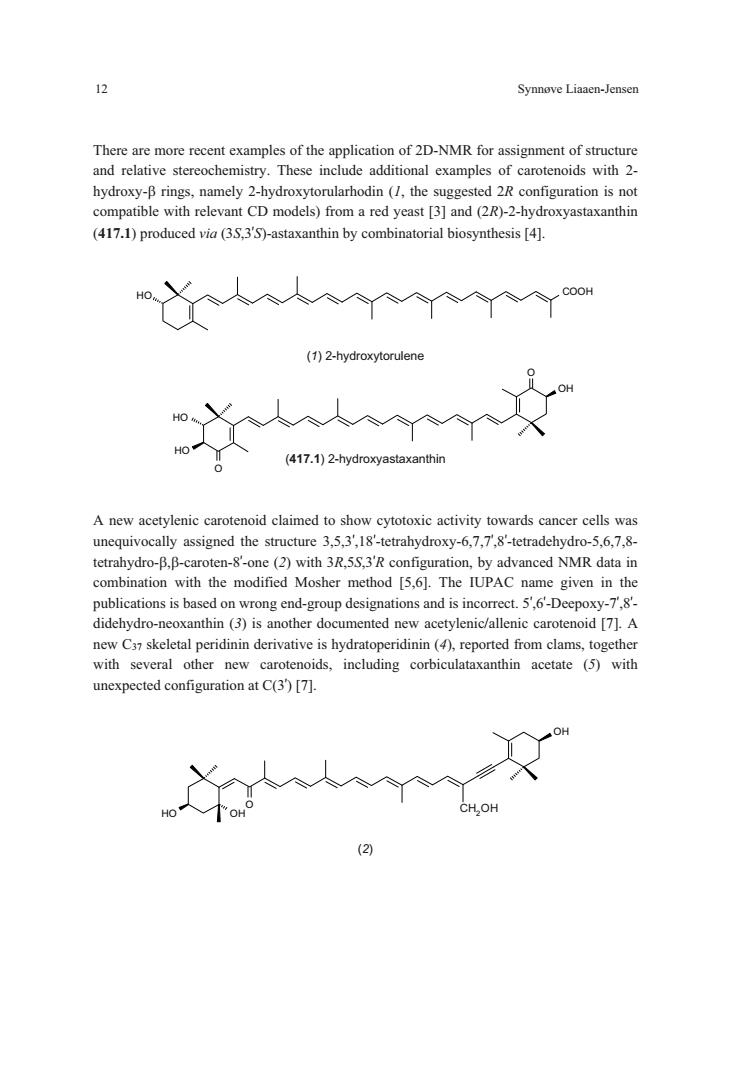
Synnove Liaaen-Jensen There are more recent examples of the application of 2D-NMR for assignment of structure and relative stereochemistry.These include additional examples of carotenoids with 2- hydrxy-Bringnamely -(the compatible with relevant CD models)from a red yeast [3]and (2R)-2-hydroxyastaxanthin (417.1)produced via (3.S,3'S)-astaxanthin by combinatorial biosynthesis [4]. 人人m (1)2-hydroxytorulene 如 (417.1)2-hydroxyastaxanthin A new acetylenic carotenoid claimed to show cytotoxic activity towards cancer cells was unequivocally assigned the structure 3.5.3.18'-tetrahydroxy-6.7.7.8'-tetradehydro-5,6.7.8- tetrahydro-B.B-caroten--one(2)with 3R.configuration,by advanced NMR data in combination with the modified Mosher method [5,6].The IUPAC name given in the publications is based on wrong end-group designations and is incorrect.5',6'-Deepoxy-7'.8'- didehydro-neoxanthin (3)is another documented new acetvlenic/allenic carotenoid 171.A with several other new carotenoids,including corbiculataxanthin acetate (5)with unexpected configuration at C(3)[7]
12 Synnøve Liaaen-Jensen OH O HO O HO There are more recent examples of the application of 2D-NMR for assignment of structure and relative stereochemistry. These include additional examples of carotenoids with 2- hydroxy- rings, namely 2-hydroxytorularhodin (1, the suggested 2R configuration is not compatible with relevant CD models) from a red yeast [3] and (2R)-2-hydroxyastaxanthin (417.1) produced via (3S,3’S)-astaxanthin by combinatorial biosynthesis [4]. HO COOH (1) 2-hydroxytorulene (417.1) 2-hydroxyastaxanthin A new acetylenic carotenoid claimed to show cytotoxic activity towards cancer cells was unequivocally assigned the structure 3,5,3’,18’-tetrahydroxy-6,7,7’,8’-tetradehydro-5,6,7,8- tetrahydro-,-caroten-8’-one (2) with 3R,5S,3’R configuration, by advanced NMR data in combination with the modified Mosher method [5,6]. The IUPAC name given in the publications is based on wrong end-group designations and is incorrect. 5’,6’-Deepoxy-7’,8’- didehydro-neoxanthin (3) is another documented new acetylenic/allenic carotenoid [7]. A new C37 skeletal peridinin derivative is hydratoperidinin (4), reported from clams, together with several other new carotenoids, including corbiculataxanthin acetate (5) with unexpected configuration at C(3’) [7]. OH OH O OH CH2OH (2) ���
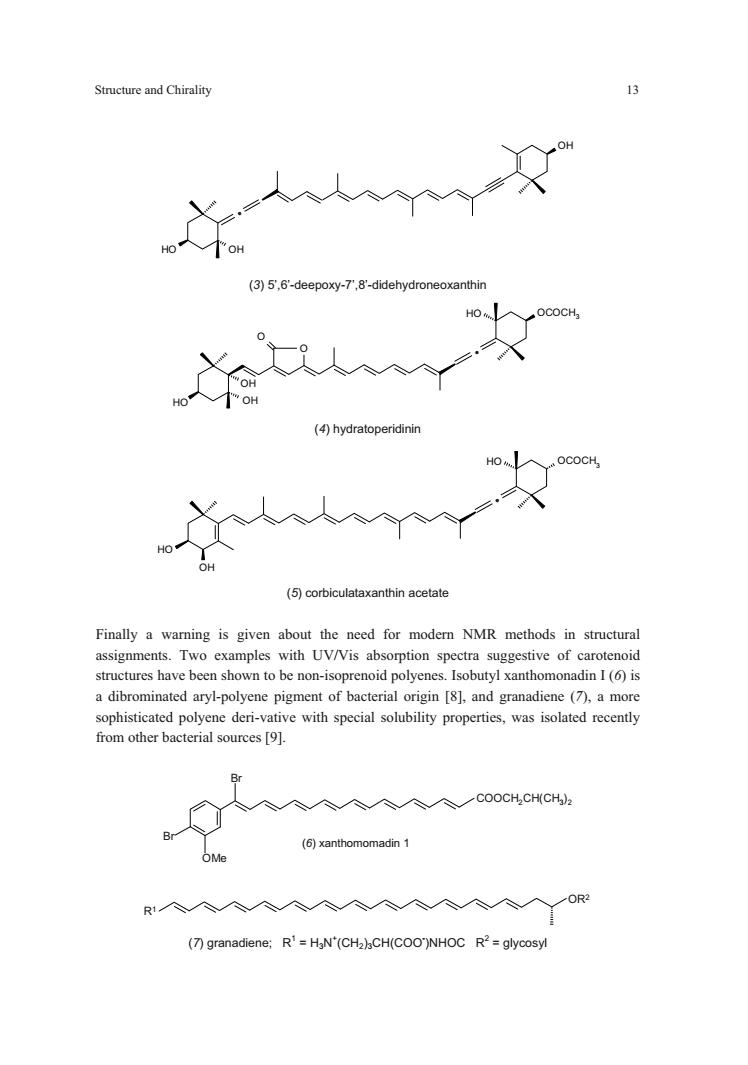
Structure and Chirality (3)5.6-deepoxy-7.8-didehydroneoxanthin (④hydratoperidinin (5)corbiculataxanthin acetate Finally a warning is given about the need for modern NMR methods in structural assignments.Two examples with UV/Vis absorption spectra suggestive of carotenoid stuctureshave tobe n-isoprepolyenes(is a dibrominated aryl-polyene pigment of bacterial origin [8],and granadiene (7),a more sophisticated polyene deri-vative with special solubility properties,was isolated recently from other bacterial sources[. 入入入入入入COOCHCHCH) (6)xanthomomadin 1 OMe R人个人人人人人人人人人人 (7)granadiene:R'=HaN'(CHa)CH(COO)NHOC R2=glycosyl
Structure and Chirality 13 OH OH OH . (3) 5’,6’-deepoxy-7’,8’-didehydroneoxanthin HO OCOCH3 OH OH O O . HO (4) hydratoperidinin HO OCOCH3 HO OH . (5) corbiculataxanthin acetate Finally a warning is given about the need for modern NMR methods in structural assignments. Two examples with UV/Vis absorption spectra suggestive of carotenoid structures have been shown to be non-isoprenoid polyenes. Isobutyl xanthomonadin I (6) is a dibrominated aryl-polyene pigment of bacterial origin [8], and granadiene (7), a more sophisticated polyene deri-vative with special solubility properties, was isolated recently from other bacterial sources [9]. Br Br OMe COOCH2CH(CH3)2 (6) xanthomomadin 1 R1 OR2 (7) granadiene; R1 = H3N+ (CH2)3CH(COO- )NHOC R2 = glycosyl

Synnove Liaaen-Jensen References [1]R.S.Cahn.J Chem.Educ.41,116(1964). [2]H.Friebolin,Basic One-and Two-Dimensional NMR Spectroscopy.3rd Edition.Wiley-VCH. Weinheim (1998). [3]R.W.S.Weber,A.Madhour,H.Anke,A.Mucci and P.Davoli.Helv.Chim.,(005) [4]Y.Nishida,K.Adachi,H.Kasai.Y.Shiguri,K.Shindo,A.Sawabe,S.Kommushi.W.Miki and N. Misawa,Appl.Environ.Mficrobiol 71,4286(2005). 5]E.W.RogersandT.F.Molinky..NaL) [6]1.Ohtani,T.Kusumi.Y.Kashman and H.Kakisawam.Chem.Soc (005). [7]T.Maoka,M.Takashi,F.Yasuhiro,H.Keiji and A.Naoshiga,J.Nat.Prod,68.1341 (2005). [8]A.G.Andrewes.C.LJenkins,M.P.Starr.J.Shepherd and H.Hope,Terrahedron Lens..4023(1976). [M.Rosa-Fraile,.Rodriguez-Granger,A.Haidoue-Benamin,J.Manuel-CuervaandA.Sampedropp/ Environ.Microbiol,72.6367(2006)
14 Synnøve Liaaen-Jensen References [1] R. S. Cahn, J. Chem. Educ., 41, 116 (1964). [2] H. Friebolin, Basic One- and Two-Dimensional NMR Spectroscopy, 3rd Edition, Wiley-VCH, Weinheim (1998). [3] R. W. S. Weber, A. Madhour, H. Anke, A. Mucci and P. Davoli, Helv. Chim. Acta, 88, 2960 (2005). [4] Y. Nishida, K. Adachi, H. Kasai, Y. Shiguri, K. Shindo, A. Sawabe, S. Kommushi, W. Miki and N. Misawa, Appl. Environ. Microbiol., 71, 4286 (2005). [5] E. W. Rogers and T. F. Molinsky, J. Nat. Prod., 68, 450 (2005). [6] I. Ohtani, T. Kusumi, Y. Kashman and H. Kakisawa, J. Am. Chem. Soc., 113, 4092 (2005). [7] T. Maoka, M. Takashi, F. Yasuhiro, H. Keiji and A. Naoshiga, J. Nat. Prod., 68, 1341 (2005). [8] A. G. Andrewes, C. L. Jenkins, M. P. Starr, J. Shepherd and H. Hope, Tetrahedron Letts., 4023 (1976). [9] M. Rosa-Fraile, J. Rodriguez-Granger, A. Haidoue-Benamin, J. Manuel-Cuerva and A. Sampedro, Appl. Environ. Microbiol., 72, 6367 (2006)