
Special Molecules,Special Properties a)Aggregation Being highly hydrophobic,carotenoids show a strong tendency to aggregate and crystallize especially in aqueous media.Aggregation changes the properties of carotenoids,e.g.light absorption and chemical reactivity,as well as their effective size and ase of solubilization In vivo,therefore,it may be such aggregates that are the biologically active or influential factors rather than the simple carotenoid monomers.The aggregation of carotenoid molecules is described in detail in Chapter5. b)Carotenoids in membranes Carotenoids are highly hydrophobic compounds which will therefore tend to be associated with lipid (oil.fat)or in hvdrophobic structures such as membranes.Hvdrophobic molecules are often located in natural membranes,and constitute an integral part of the complex membrane structure.Predictably,carotenoids are associated with the lipid core of the membrane bilayer,but the concentration that can be achieved,the orientation of a particular carotenoid in the membrane,and its effect on membrane properties,depend on structural features such as the size and shape of the carotenoid and the presence of functional groups The topic of carotenoids in membranes is treated in Chapter 10,and the aggregation of carotenoids in membranes is addressed in Chapter5. )Carotenoid-protein interactions Interactions between carotenoids and proteins occur in all kinds of living organisms Carotenoids are usually much more stable inviv than they are after isolation.The interactions can alter the chemical or physical properties of the carotenoid.A striking example of this is provided by the blue carotenoproteins found in many invertebrate animals. These arotenoproteins and other examples of crotnd-rotein interactionsare discussed in detail in Chapter 6,including associations with blood lipoproteins for carotenoid transport The structures of the photosynthetic pigment-protein complexes that maintain correct between carotenoid and chlorophyll,are described in Chapter C.Functions of Carotenoids 4 then describes the main functions ascribed to carotenoids.Chapter /0 gives an overview of the main known and proposed functions of intact carotenoids,including the contribution that carotenoids make to natural colouration along with other pigments and structural colours
Special Molecules, Special Properties 5 a) Aggregation Being highly hydrophobic, carotenoids show a strong tendency to aggregate and crystallize, especially in aqueous media. Aggregation changes the properties of carotenoids, e.g. light absorption and chemical reactivity, as well as their effective size and ease of solubilization. In vivo, therefore, it may be such aggregates that are the biologically active or influential factors rather than the simple carotenoid monomers. The aggregation of carotenoid molecules is described in detail in Chapter 5. b) Carotenoids in membranes Carotenoids are highly hydrophobic compounds which will therefore tend to be associated with lipid (oil, fat) or in hydrophobic structures such as membranes. Hydrophobic molecules are often located in natural membranes, and constitute an integral part of the complex membrane structure. Predictably, carotenoids are associated with the lipid core of the membrane bilayer, but the concentration that can be achieved, the orientation of a particular carotenoid in the membrane, and its effect on membrane properties, depend on structural features such as the size and shape of the carotenoid and the presence of functional groups. The topic of carotenoids in membranes is treated in Chapter 10, and the aggregation of carotenoids in membranes is addressed in Chapter 5. c) Carotenoid-protein interactions Interactions between carotenoids and proteins occur in all kinds of living organisms. Carotenoids are usually much more stable in vivo than they are after isolation. The interactions can alter the chemical or physical properties of the carotenoid. A striking example of this is provided by the blue carotenoproteins found in many invertebrate animals. These carotenoproteins and other examples of carotenoid-protein interactions are discussed in detail in Chapter 6, including associations with blood lipoproteins for carotenoid transport. The structures of the photosynthetic pigment-protein complexes that maintain correct positioning of carotenoids with respect to other moleclues, and allow efficient energy transfer between carotenoid and chlorophyll, are described in Chapter 14. C. Functions of Carotenoids With the fundamental principles of structure and properties in mind, the latter part of Volume 4 then describes the main functions ascribed to carotenoids. Chapter 10 gives an overview of the main known and proposed functions of intact carotenoids, including the contribution that carotenoids make to natural colouration along with other pigments and structural colours

6 George Britton,Synnove Liaaen-Jensen and Hanspeter Pfander effects on membrane structure and properties,and various 'photofunctions'such as by carotenoids from the perspective of behavioural science and ecology.Commercial applications of carotenoids as colourants in aquaculture and poultry production are then evaluated in Chapters 12 and 13. The roles of carotenoids in photosynthesis are discussed in detail in Chapter 14.This advanced treatment shows what can be achieved by the application of a battery of powerful spectroscopic and other physical techniques. D.Metabolites and Breakdown Products Any discussion of the biological significance of carotenoids must not overlook the role of carotenoids as precursors of metabolites that have important biological roles and of breakdown products that could be the actual biologically active molecules in functions and actions attributed to the carotenoids themselves.The roles of vitamin A and its derivatives in vision.cellular regulation and hormone action have been known for a long time.There is now increasing interest in the role of carotenoid fragments (norisoprenoids)as highly effective components of natural perfumes and flower fragrances and of flavour/aroma of tea wine ete.Functions of carotenoid metabolites and fragments are surveyed in Chapter 15,and the importance of vitaminA and retinoids in human health is treated in Vome5.Chapters8 and 9.The enzymic formation of retinoids and other norisoprenoids is described in Chapters 16 and 17,respectively.The extensive treatment of carotenoids and human health in Volume 5 at times alludes to the possibility that some of the biological effects seen could be attributable to breakdown products.This possibility is evaluated. E.Conclusions its companion,which deals with human utrition and health,are the last volumes in the Carofenoids series.and in many ways point the way to the future of carotenoid research.They highlight how biologists are not only discovering new phenomena, but are looking at functions in a new light,and striving to elucidate details of the underlying mechanisms that explain their observations.They also show how chemistry is moving in new directions relevant to studies in vivo of biological actions and how new techniques are being developed to study these increasingly complex and sophisticated systems.If the insight that these books provide stimulates chemists,physicists and biologists to understand and talk to each other and thus provides a catalyst for interdisciplinary studies that will bring great advances and rewards in the future,then the editors will feel that their time and effort has been well spent
6 George Britton, Synnøve Liaaen-Jensen and Hanspeter Pfander effects on membrane structure and properties, and various ‘photofunctions’ such as photoprotection. Chapter 11 takes a novel approach, assessing the significance of colouration by carotenoids from the perspective of behavioural science and ecology. Commercial applications of carotenoids as colourants in aquaculture and poultry production are then evaluated in Chapters 12 and 13. The roles of carotenoids in photosynthesis are discussed in detail in Chapter 14. This advanced treatment shows what can be achieved by the application of a battery of powerful spectroscopic and other physical techniques. D. Metabolites and Breakdown Products Any discussion of the biological significance of carotenoids must not overlook the role of carotenoids as precursors of metabolites that have important biological roles and of breakdown products that could be the actual biologically active molecules in functions and actions attributed to the carotenoids themselves. The roles of vitamin A and its derivatives in vision, cellular regulation and hormone action have been known for a long time. There is now increasing interest in the role of carotenoid fragments (norisoprenoids) as highly effective components of natural perfumes and flower fragrances and of flavour/aroma of tea, wine etc. Functions of carotenoid metabolites and fragments are surveyed in Chapter 15, and the importance of vitamin A and retinoids in human health is treated in Volume 5, Chapters 8 and 9. The enzymic formation of retinoids and other norisoprenoids is described in Chapters 16 and 17, respectively. The extensive treatment of carotenoids and human health in Volume 5 at times alludes to the possibility that some of the biological effects seen could be attributable to breakdown products. This possibility is evaluated in Volume 5, Chapter 18. E. Conclusions Volume 4 and its companion, Volume 5, which deals with human nutrition and health, are the last volumes in the Carotenoids series, and in many ways point the way to the future of carotenoid research. They highlight how biologists are not only discovering new phenomena, but are looking at functions in a new light, and striving to elucidate details of the underlying mechanisms that explain their observations. They also show how chemistry is moving in new directions relevant to studies in vivo of biological actions and how new techniques are being developed to study these increasingly complex and sophisticated systems. If the insight that these books provide stimulates chemists, physicists and biologists to understand and talk to each other and thus provides a catalyst for interdisciplinary studies that will bring great advances and rewards in the future, then the editors will feel that their time and effort has been well spent

2008 Birkhauser Verlag Basel Chapter 2 Structure and Chirality Synnove Liaaen-Jensen A.Introduction Naturally occurring carotenoids display considerable structural diversity,including different carbon skeletons,various types of oxygen functions and variable degree of unsaturation(double-bond equivalents)due to cyclization,double,triple or allenic bonds. In the Carotenoids Handbook the structures and properties of the known naturally occurring carotenoids (published up to 2003)are presented in two groups,(i)those considered to be proved unequivocally (Main List,some 500 compounds)and(ii)those requiring additional supporting evidence or proof of natural occurrence(Supplementary List,some 25 compounds) B.Three-dimensional Carotenoid Structures A feature often overlooked but crucial to the localization and functioning of carotenoids in biological systems is their 3-dimensional shape,especially chirality.Even achiral carotenoids are -dimensional molecules.Around a quarter of the known carotenoids are achiral,the majority chiral.Achiral carotenoids are encountered particularly among C carotenes and their apo-carotenoids,C3o diapocarotenoids and acyclic xanthophylls (oxygenated carotenoids)from phototrophic and some other bacteria. carotenoids,which are different from their mirror image and contain chiral centres or a
Carotenoids Volume 4: Natural Functions © 2008 Birkhäuser Verlag Basel Chapter 2 Structure and Chirality Synnøve Liaaen-Jensen A. Introduction Naturally occurring carotenoids display considerable structural diversity, including different carbon skeletons, various types of oxygen functions and variable degree of unsaturation (double-bond equivalents) due to cyclization, double, triple or allenic bonds. In the Carotenoids Handbook the structures and properties of the known naturally occurring carotenoids (published up to 2003) are presented in two groups, (i) those considered to be proved unequivocally (Main List, some 500 compounds) and (ii) those requiring additional supporting evidence or proof of natural occurrence (Supplementary List, some 225 compounds). B. Three-dimensional Carotenoid Structures A feature often overlooked but crucial to the localization and functioning of carotenoids in biological systems is their 3-dimensional shape, especially chirality. Even achiral carotenoids are 3-dimensional molecules. Around a quarter of the known carotenoids are achiral, the majority chiral. Achiral carotenoids are encountered particularly among C40 carotenes and their apo-carotenoids, C30 diapocarotenoids and acyclic xanthophylls (oxygenated carotenoids) from phototrophic and some other bacteria. Achiral carotenoids are identical with their own mirror image, in contrast to the chiral carotenoids, which are different from their mirror image and contain chiral centres or a
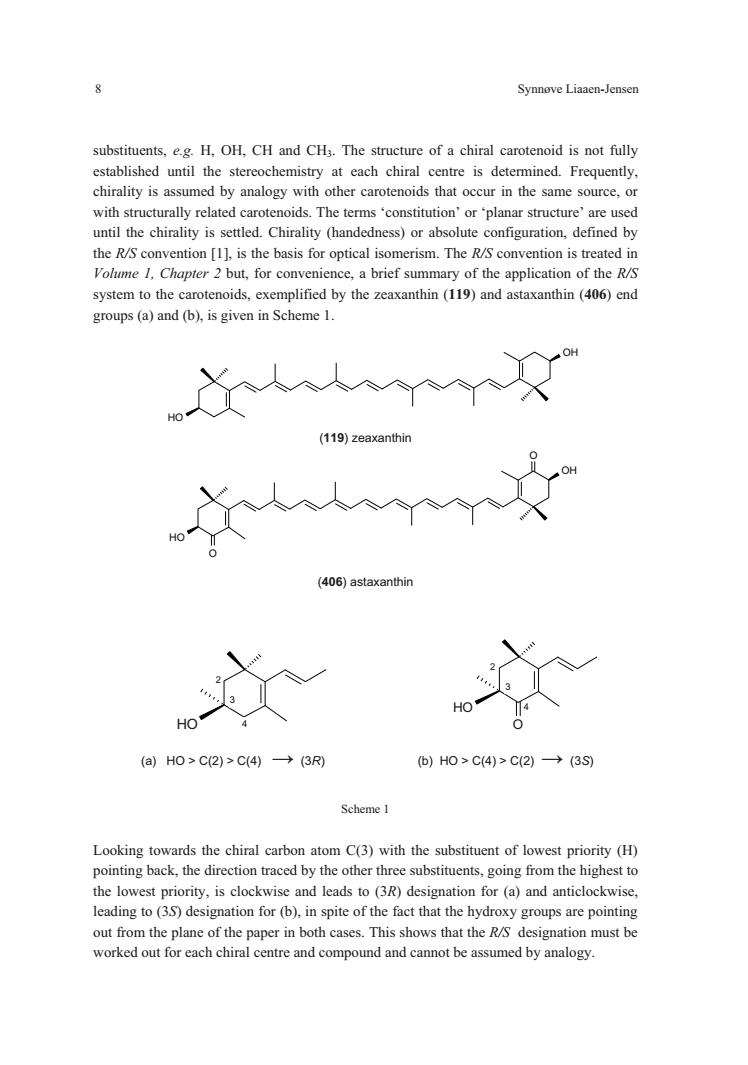
Synnove Liaaen-Jensen substituents,e.g.H,OH,CH and CH3.The structure of a chiral carotenoid is not fully chirality is assumed by analogy with other carotenoids that occur in the same source,or with structurally related carotenoids.The terms'constitution'or'planar structure'are used until the chirality is settled.Chirality (handedness)or absolute configuration,defined by system to the carotenoids,exemplified by the zeaxanthin(119)and astaxanthin(406)end groups(a)and(b),is given in Scheme 1. (119)zeaxanthi (406)astaxanthin (a)Ho>C2)>C(4→(3R )H0>C4)>C2)→(3S Looking towards the chiral carbon atom C(3)with the substituent of lowest priority (H) pointing back,the direction traced by the other three substituents,going from the highest to the lowest priority,is clockwise and leads to (3R)designation for (a)and anticlockwise. leading to (3S)designation for(b),in spite of the fact that the hydroxy groups are pointing out from the plane of the paper in both cases.This shows that the R/S designation must be worked out for each chiral centre and compound and cannot be assumed by analogy
8 Synnøve Liaaen-Jensen HO OH OH O OH O substituents, e.g. H, OH, CH and CH3. The structure of a chiral carotenoid is not fully established until the stereochemistry at each chiral centre is determined. Frequently, chirality is assumed by analogy with other carotenoids that occur in the same source, or with structurally related carotenoids. The terms ‘constitution’ or ‘planar structure’ are used until the chirality is settled. Chirality (handedness) or absolute configuration, defined by the R/S convention [1], is the basis for optical isomerism. The R/S convention is treated in Volume 1, Chapter 2 but, for convenience, a brief summary of the application of the R/S system to the carotenoids, exemplified by the zeaxanthin (119) and astaxanthin (406) end groups (a) and (b), is given in Scheme 1. (119) zeaxanthin (406) astaxanthin OH 2 3 4 OH O 2 3 4 (a) HO > C(2) > C(4) (3R) (b) HO > C(4) > C(2) (3S) Scheme 1 Looking towards the chiral carbon atom C(3) with the substituent of lowest priority (H) pointing back, the direction traced by the other three substituents, going from the highest to the lowest priority, is clockwise and leads to (3R) designation for (a) and anticlockwise, leading to (3S) designation for (b), in spite of the fact that the hydroxy groups are pointing out from the plane of the paper in both cases. This shows that the R/S designation must be worked out for each chiral centre and compound and cannot be assumed by analogy. ��
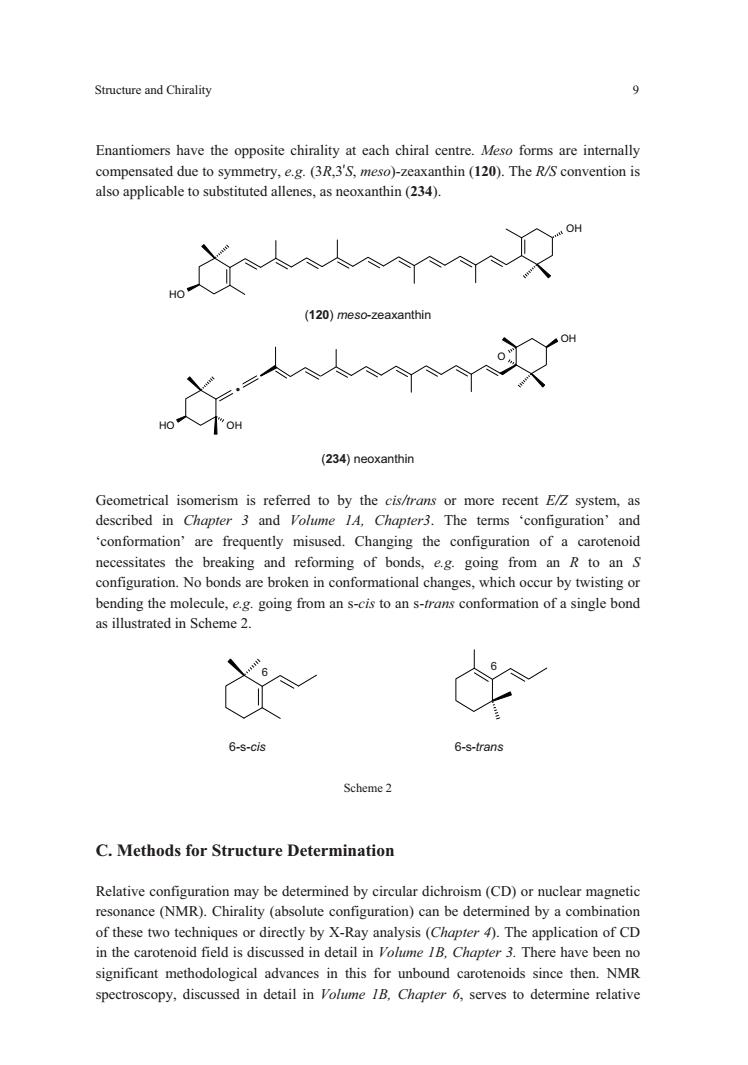
Structure and Chirality 9 Enantiomrs have the opposite chirality at each chiral formsare intemaly compensated due to symmetry,e.g.(3R,3'S,meso)-zeaxanthin(120).The R/S convention is also applicable to substituted allenes,as neoxanthin(234). 人人父 120)meso-zeaxanth (234)neoxanthin Geometrical isomerism is referred to by the cis/trans or more recent EZ system,as described in Chapter 3 and Volumne lA.Chapter3.The terms 'configuration'and frequently misused.Changing the of a carotenoid necessitates the breaking and reforming of bonds,e.g.going from an R to an S configuration.No bonds are broken in conformational changes,which occur by twisting or 6 6-s-cis 6-s-trans Scheme2 C.Methods for Structure Determination Relative configuration may be determined by circular dichroism(CD)or nuclear magnetic resonance (NMR).Chirality (absolute configuration)can be determined by a combination of these two techniques or directly by X-Ray analysis (Chapter).The application of CD in the carotenoid field is discussed in detail in Volume IB.Chapter 3.There have been no significant methodological advances in this for unbound carotenoids since then.NMR spectroscopy,discussed in detail in olme 1B.Chapter6,serves to determine relative
Structure and Chirality 9 OH OH Enantiomers have the opposite chirality at each chiral centre. Meso forms are internally compensated due to symmetry, e.g. (3R,3’S, meso)-zeaxanthin (120). The R/S convention is also applicable to substituted allenes, as neoxanthin (234). (120) meso-zeaxanthin O OH OH OH . (234) neoxanthin Geometrical isomerism is referred to by the cis/trans or more recent E/Z system, as described in Chapter 3 and Volume 1A, Chapter3. The terms ‘configuration’ and ‘conformation’ are frequently misused. Changing the configuration of a carotenoid necessitates the breaking and reforming of bonds, e.g. going from an R to an S configuration. No bonds are broken in conformational changes, which occur by twisting or bending the molecule, e.g. going from an s-cis to an s-trans conformation of a single bond as illustrated in Scheme 2. 6 6 6-s-cis 6-s-trans Scheme 2 C. Methods for Structure Determination Relative configuration may be determined by circular dichroism (CD) or nuclear magnetic resonance (NMR). Chirality (absolute configuration) can be determined by a combination of these two techniques or directly by X-Ray analysis (Chapter 4). The application of CD in the carotenoid field is discussed in detail in Volume 1B, Chapter 3. There have been no significant methodological advances in this for unbound carotenoids since then. NMR spectroscopy, discussed in detail in Volume 1B, Chapter 6, serves to determine relative