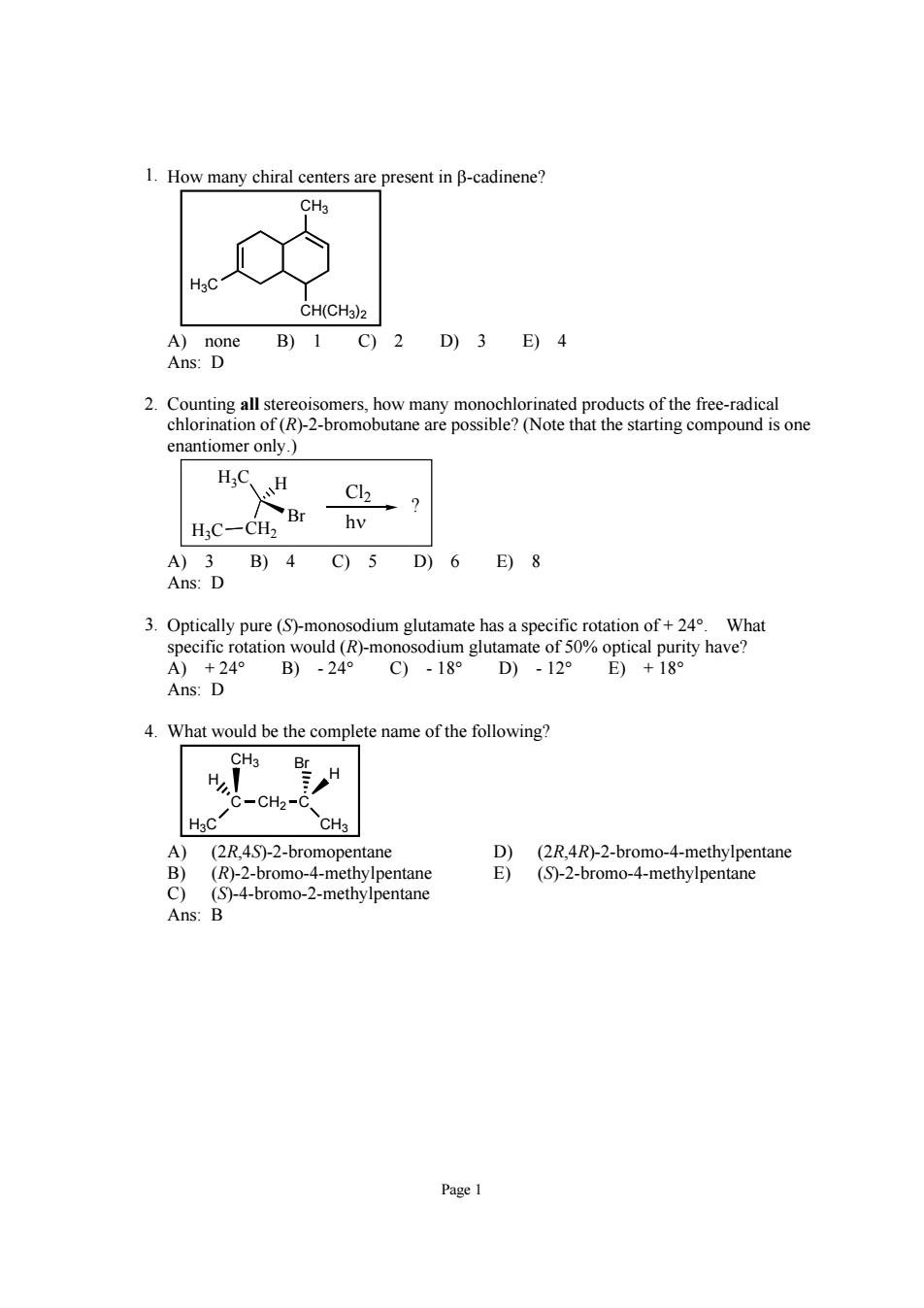
How many chiral centers are present in B-cadinene? CH3 CH(CHs)2 B)1 C)2 D)3 E)4 enantiomer only.) H,C、H Hc-ch? D B)4 C)5 D)6 E)8 3.Optically pure (S-monosodium glutamate has a specific rotation of+24What +24° B)-24° C)-18 Ans:D 4.What would be the complete name of the following? H -CH2- Hsc CH3 (2R,4S)-2-bromopentane (S)-2-bromo-4-methylpentane Ans: Page 1
Page 1 1. How many chiral centers are present in β-cadinene? CH3 CH(CH3)2 H3C A) none B) 1 C) 2 D) 3 E) 4 Ans: D 2. Counting all stereoisomers, how many monochlorinated products of the free-radical chlorination of (R)-2-bromobutane are possible? (Note that the starting compound is one enantiomer only.) H3C H3C CH2 Br H Cl2 hν ? A) 3 B) 4 C) 5 D) 6 E) 8 Ans: D 3. Optically pure (S)-monosodium glutamate has a specific rotation of + 24°. What specific rotation would (R)-monosodium glutamate of 50% optical purity have? A) + 24° B) - 24° C) - 18° D) - 12° E) + 18° Ans: D 4. What would be the complete name of the following? H3C C CH2 C CH3 CH3 H Br H A) (2R,4S)-2-bromopentane D) (2R,4R)-2-bromo-4-methylpentane B) (R)-2-bromo-4-methylpentane E) (S)-2-bromo-4-methylpentane C) (S)-4-bromo-2-methylpentane Ans: B

5.How many total stereoisomers of the following are possible? I HO OH 0 OH HOH A)1B)2C)3D)4E)6 Ans:D 6.How many total stereoisomers of the following are possible OH CO,H B)2C)3 D)4E)6 7.How many total stereoisomers of the following are possible? H HO OH o OH HO A)1B)2C)3D)4E)6 Ans:C Page2
Page 2 5. How many total stereoisomers of the following are possible? O O OH HO OH H H HO H A) 1 B) 2 C) 3 D) 4 E) 6 Ans: D 6. How many total stereoisomers of the following are possible? HO2C CO2H OH CO2H A) 1 B) 2 C) 3 D) 4 E) 6 Ans: A 7. How many total stereoisomers of the following are possible? O O OH HO H HO OH H A) 1 B) 2 C) 3 D) 4 E) 6 Ans: C
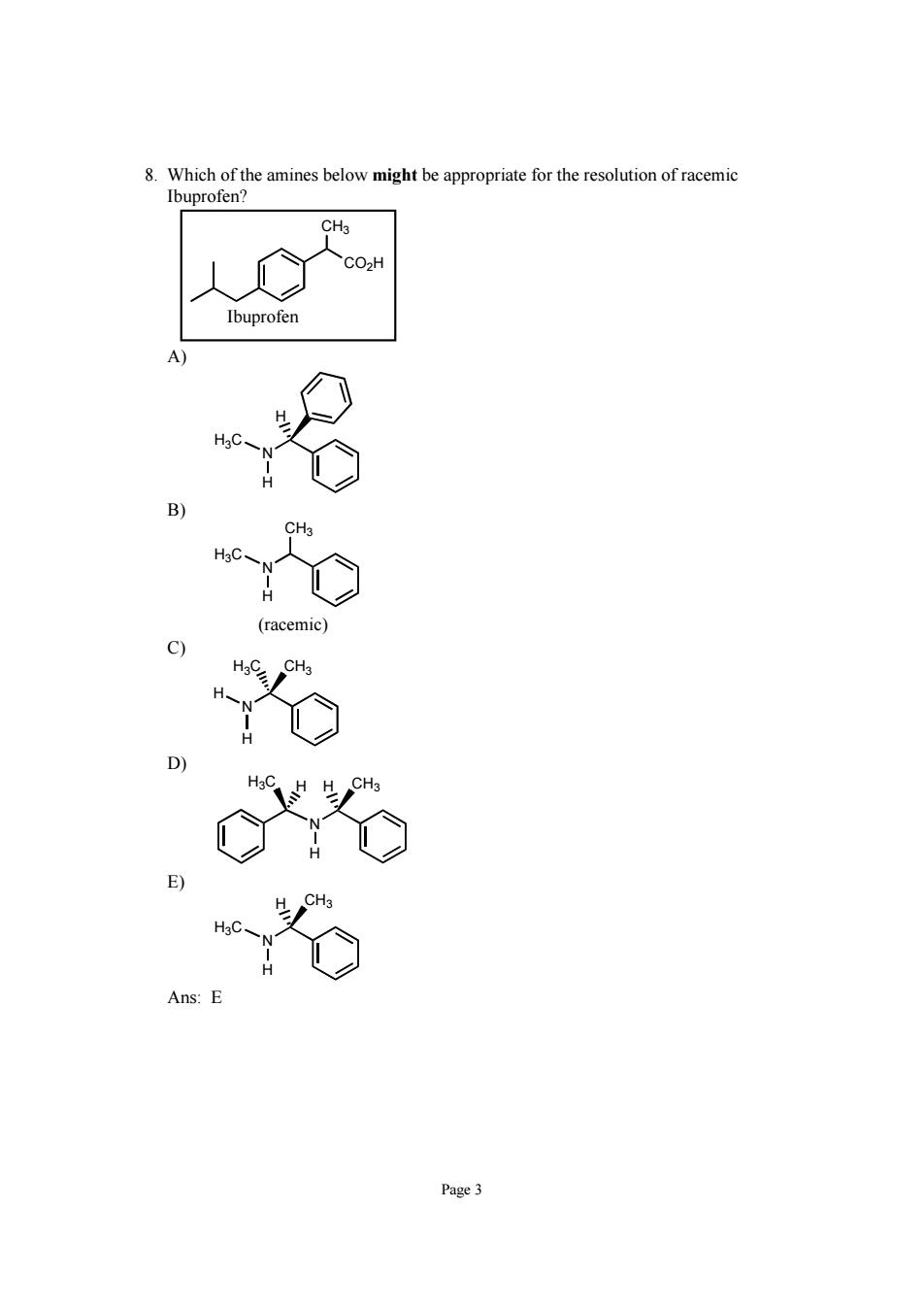
hamines below might be appropriate for the resoion of acemi 人C Page3
Page 3 8. Which of the amines below might be appropriate for the resolution of racemic Ibuprofen? CO2H CH3 Ibuprofen A) H3C N H H B) H3C N H CH3 (racemic) C) H N H H3C CH3 D) N H H3C H H CH3 E) H3C N H H CH3 Ans: E
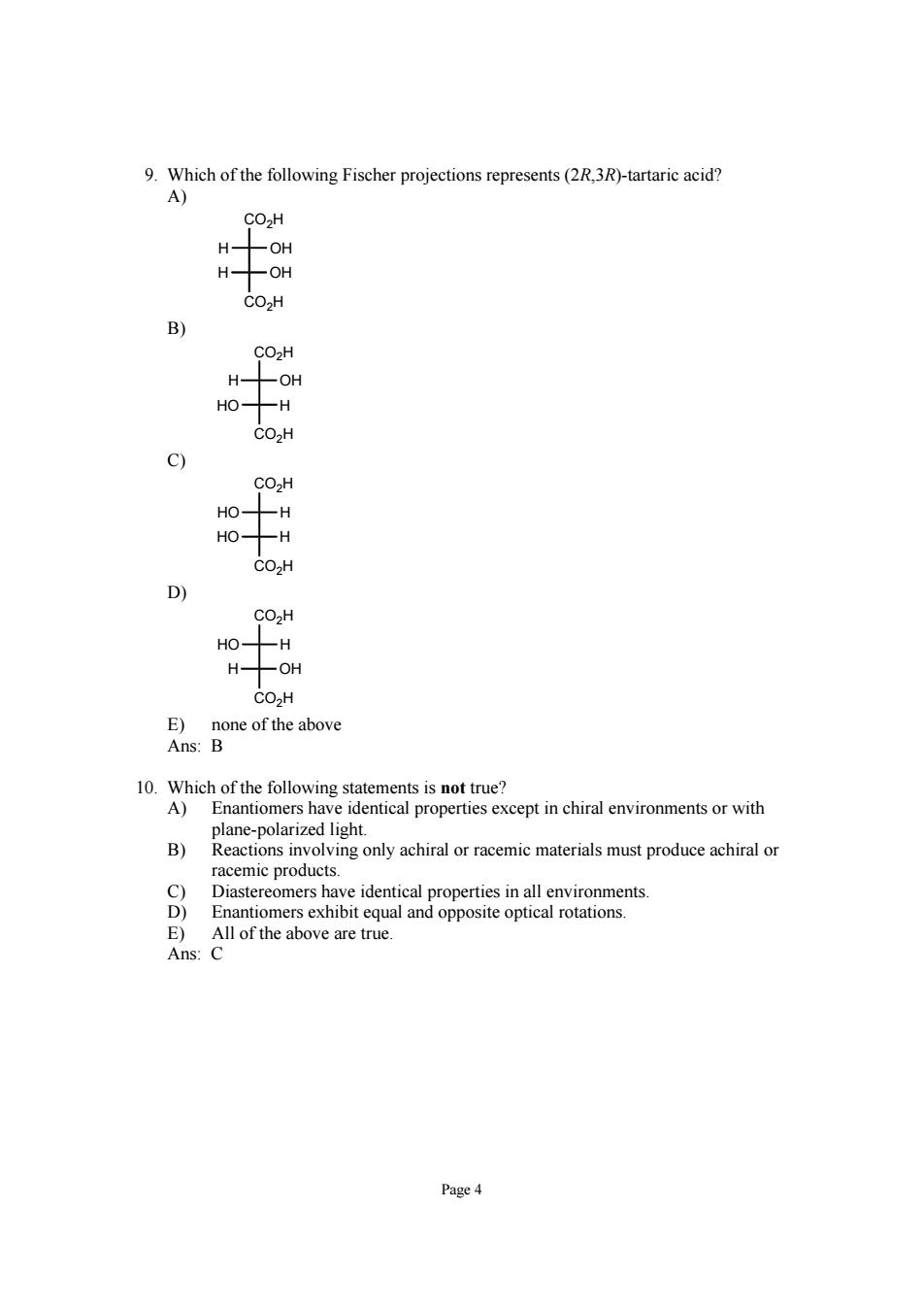
9.Which of the following Fischer projections represents(2R.3R)-tartaric acid? A) CO2H COZH B) CO2H -OH 0H CO2H CO2H CO2H CO2H -H H- OH 10.Which of the following statements is not true? A)Enantiomers have identical properties except in chiral environments or with B) 8 Enantiomers exhibit equal and opposite optical rotations. E)All of the above are true. Ans:C Page4
Page 4 9. Which of the following Fischer projections represents (2R,3R)-tartaric acid? A) CO2H CO2H H OH H OH B) CO2H CO2H HO H H OH C) CO2H CO2H HO H HO H D) CO2H CO2H H OH HO H E) none of the above Ans: B 10. Which of the following statements is not true? A) Enantiomers have identical properties except in chiral environments or with plane-polarized light. B) Reactions involving only achiral or racemic materials must produce achiral or racemic products. C) Diastereomers have identical properties in all environments. D) Enantiomers exhibit equal and opposite optical rotations. E) All of the above are true. Ans: C
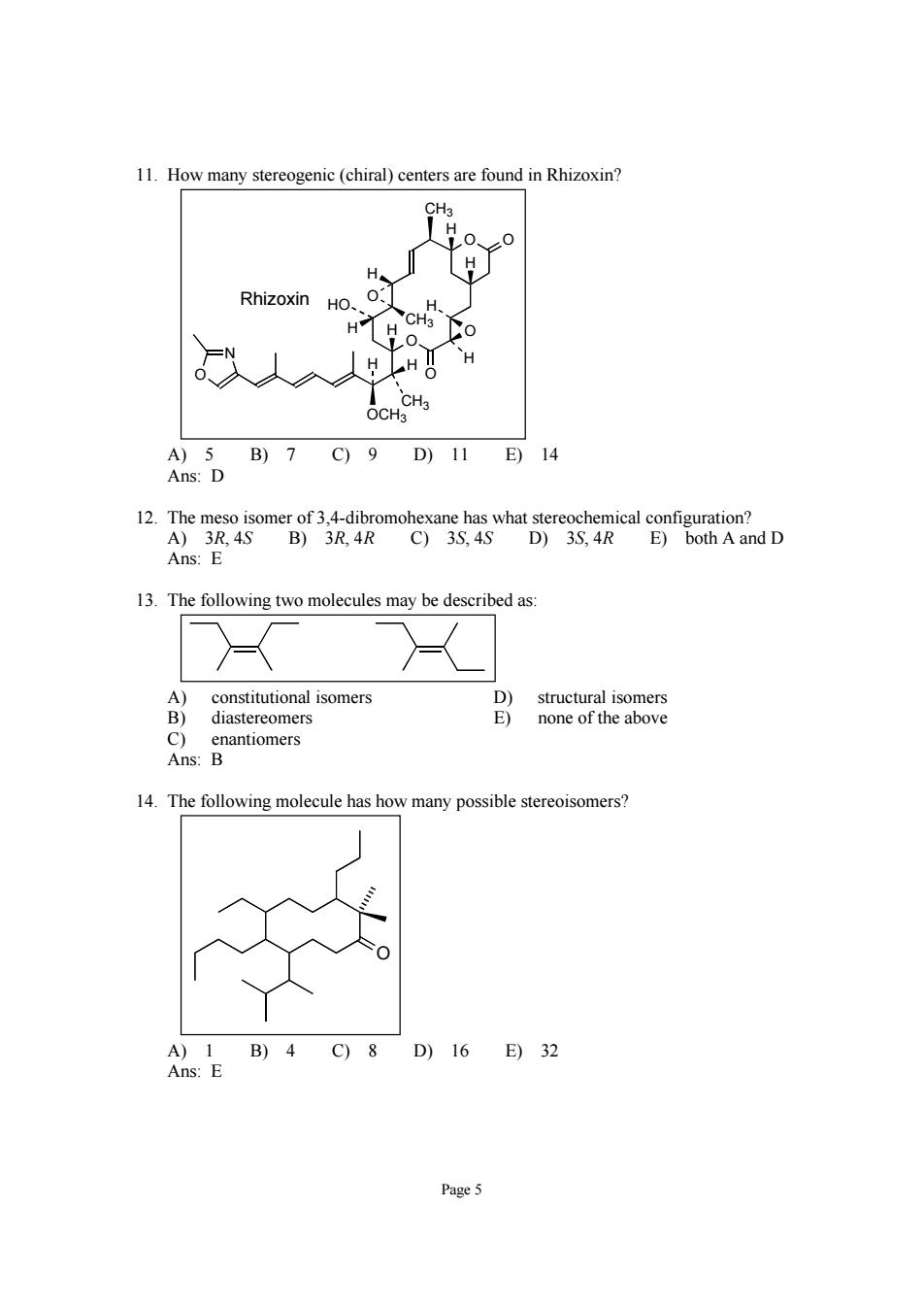
11.How many stereogenic (chiral)centers are found in Rhizoxin? Rhizoxin HO.0:] 义人 H A)5B)7C)9D)11E)14 Ans:D Ans:E 13.The following two molecules may be described as: 8mdonos 14.The following molecule has how many possible stereoisomers? B)4C)8 16 E)32 Page5
Page 5 11. How many stereogenic (chiral) centers are found in Rhizoxin? O O O O O CH3 H H O H H CH3 H HO H H CH3 H OCH3 H N O Rhizoxin A) 5 B) 7 C) 9 D) 11 E) 14 Ans: D 12. The meso isomer of 3,4-dibromohexane has what stereochemical configuration? A) 3R, 4S B) 3R, 4R C) 3S, 4S D) 3S, 4R E) both A and D Ans: E 13. The following two molecules may be described as: A) constitutional isomers D) structural isomers B) diastereomers E) none of the above C) enantiomers Ans: B 14. The following molecule has how many possible stereoisomers? O A) 1 B) 4 C) 8 D) 16 E) 32 Ans: E