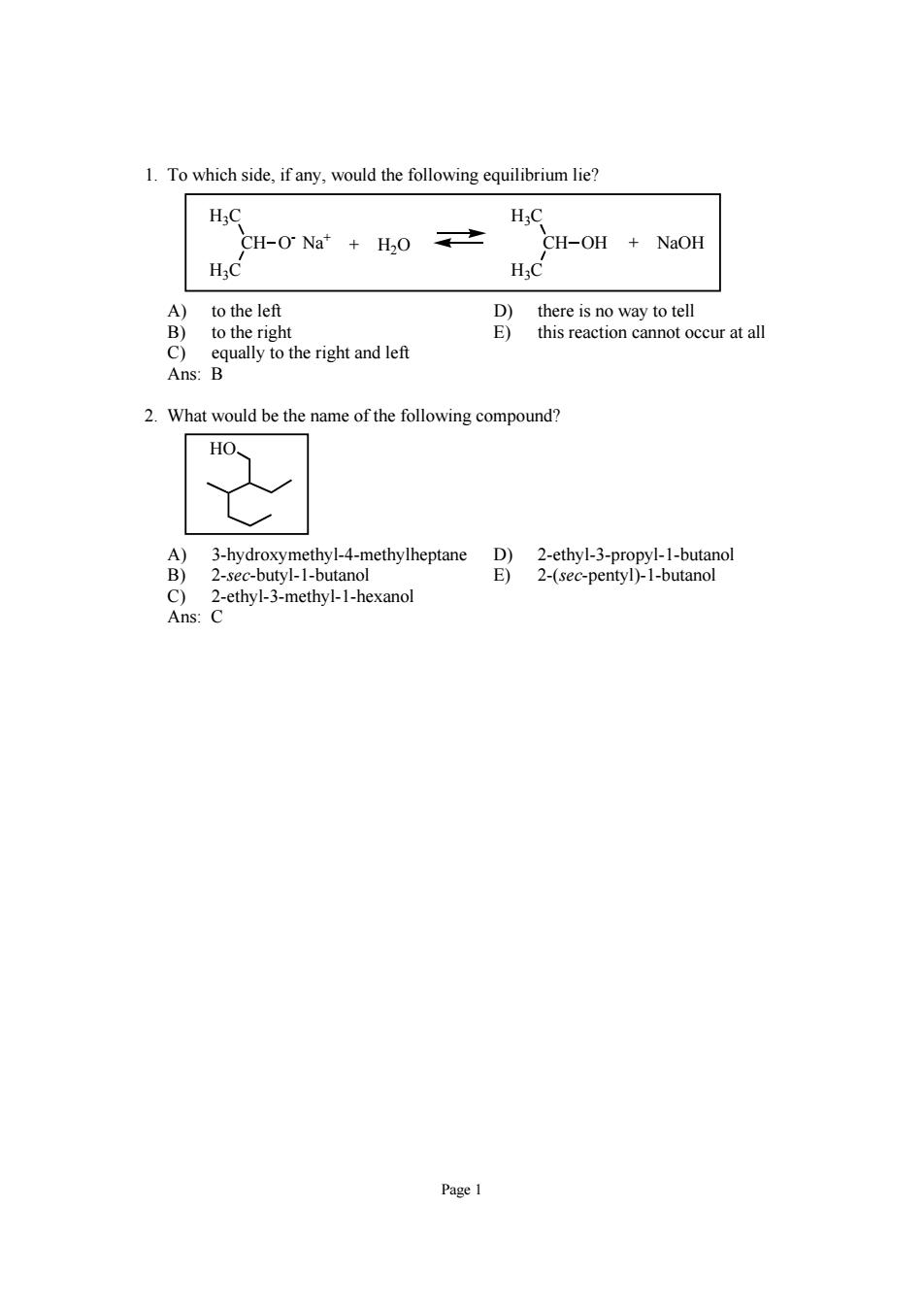
To which side,if any,would the following equilibrium lie? c H; CH-O Na"H2O 之 CH-OH NaOH A)to the left B)to the right Btsonegom this reaction cannot occur at all c Ans ul to the righ and le 2.What would be the name of the following compound? HO 2-sec-butyl-1-butanol 2-(sec-pentyl)-1-butanol thyl-3-methyl-I-hexano C
Page 1 1. To which side, if any, would the following equilibrium lie? H3C CH H3C OH H3C CH H3C O- Na+ + H2O + NaOH A) to the left D) there is no way to tell B) to the right E) this reaction cannot occur at all C) equally to the right and left Ans: B 2. What would be the name of the following compound? HO A) 3-hydroxymethyl-4-methylheptane D) 2-ethyl-3-propyl-1-butanol B) 2-sec-butyl-1-butanol E) 2-(sec-pentyl)-1-butanol C) 2-ethyl-3-methyl-1-hexanol Ans: C
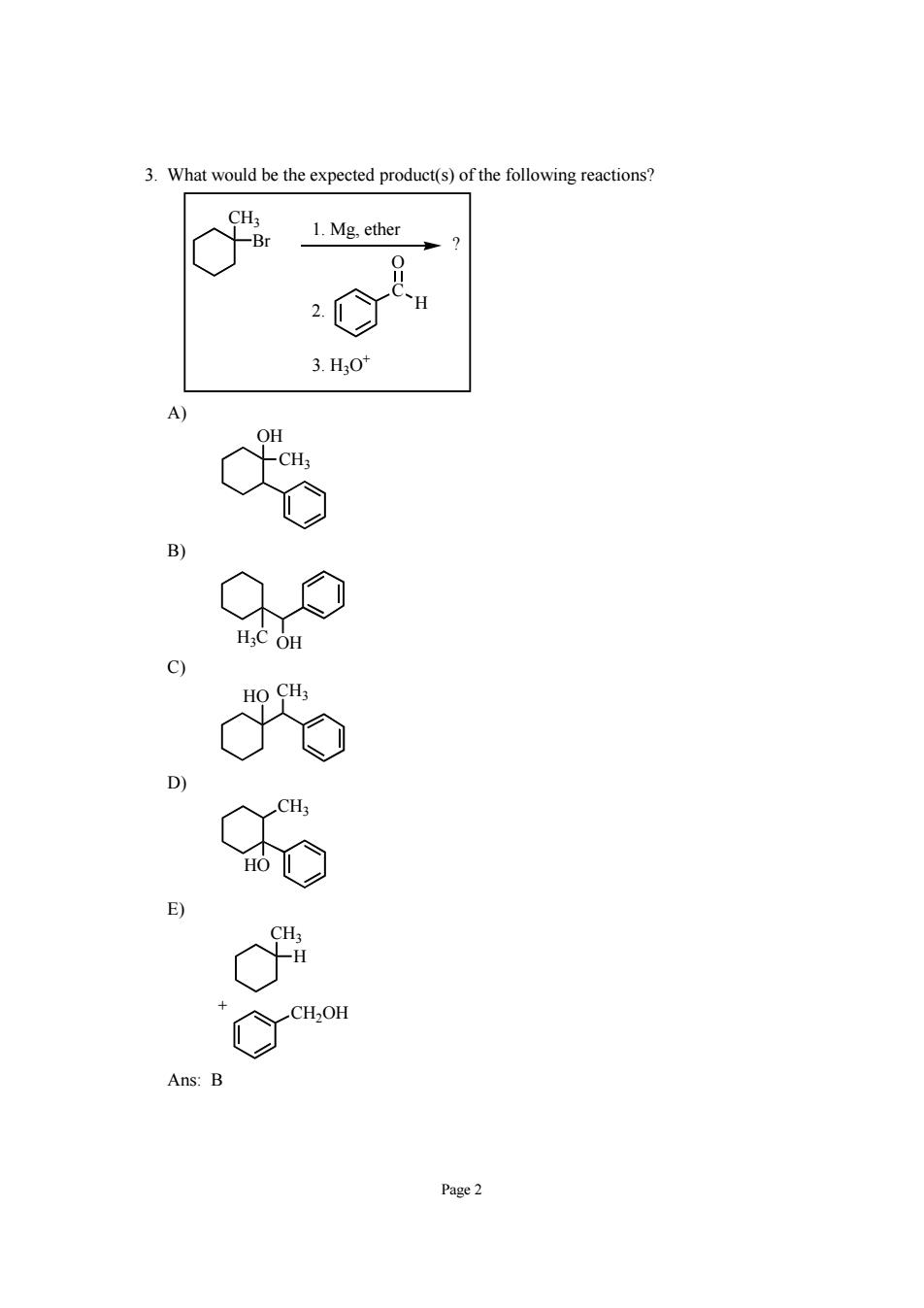
3.What would be the expected product(s)of the following reactions? o% 1.Mg.ether 3.H30 Page2
Page 2 3. What would be the expected product(s) of the following reactions? CH3 Br C O H ? 1. Mg, ether 2. 3. H3O+ A) CH3 OH B) H3C OH C) HO CH3 D) CH3 HO E) CH3 H CH2OH + Ans: B
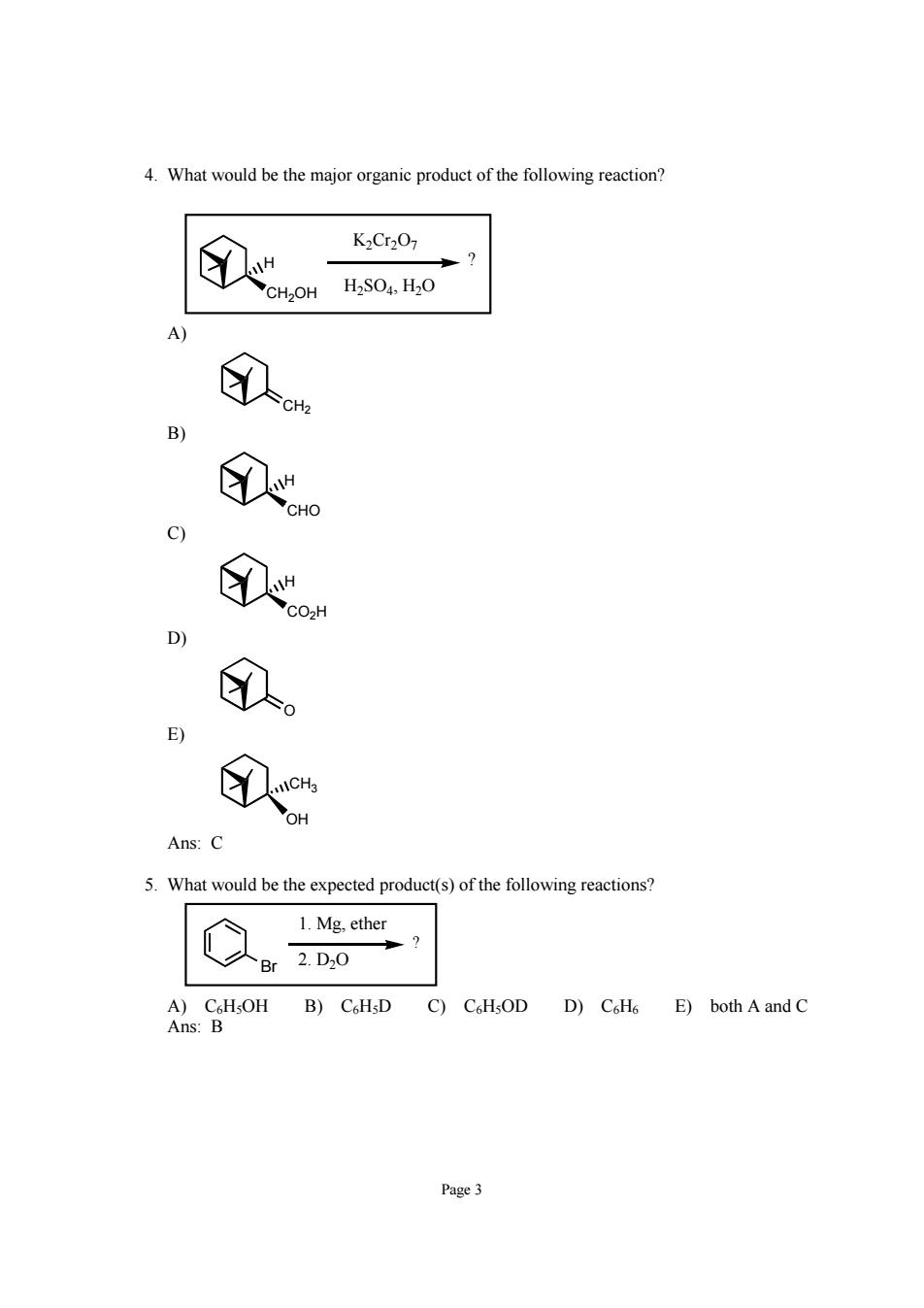
4.What would be the major organic product of the following reaction? 8 K2Cr207 A ①n ①o 9 ①o D) 。 E) ① Ans:C 5.What would be the expected product(s)ofthe following reactions? 1.Mg.ether 人Br2.D0 Ans B)CH.D D)CoH6 E)both A and C Page3
Page 3 4. What would be the major organic product of the following reaction? CH2OH H ? K2Cr2O7 H2SO4, H2O A) CH2 B) CHO H C) CO2H H D) O E) CH3 OH Ans: C 5. What would be the expected product(s) of the following reactions? Br ? 1. Mg, ether 2. D2O A) C6H5OH B) C6H5D C) C6H5OD D) C6H6 E) both A and C Ans: B
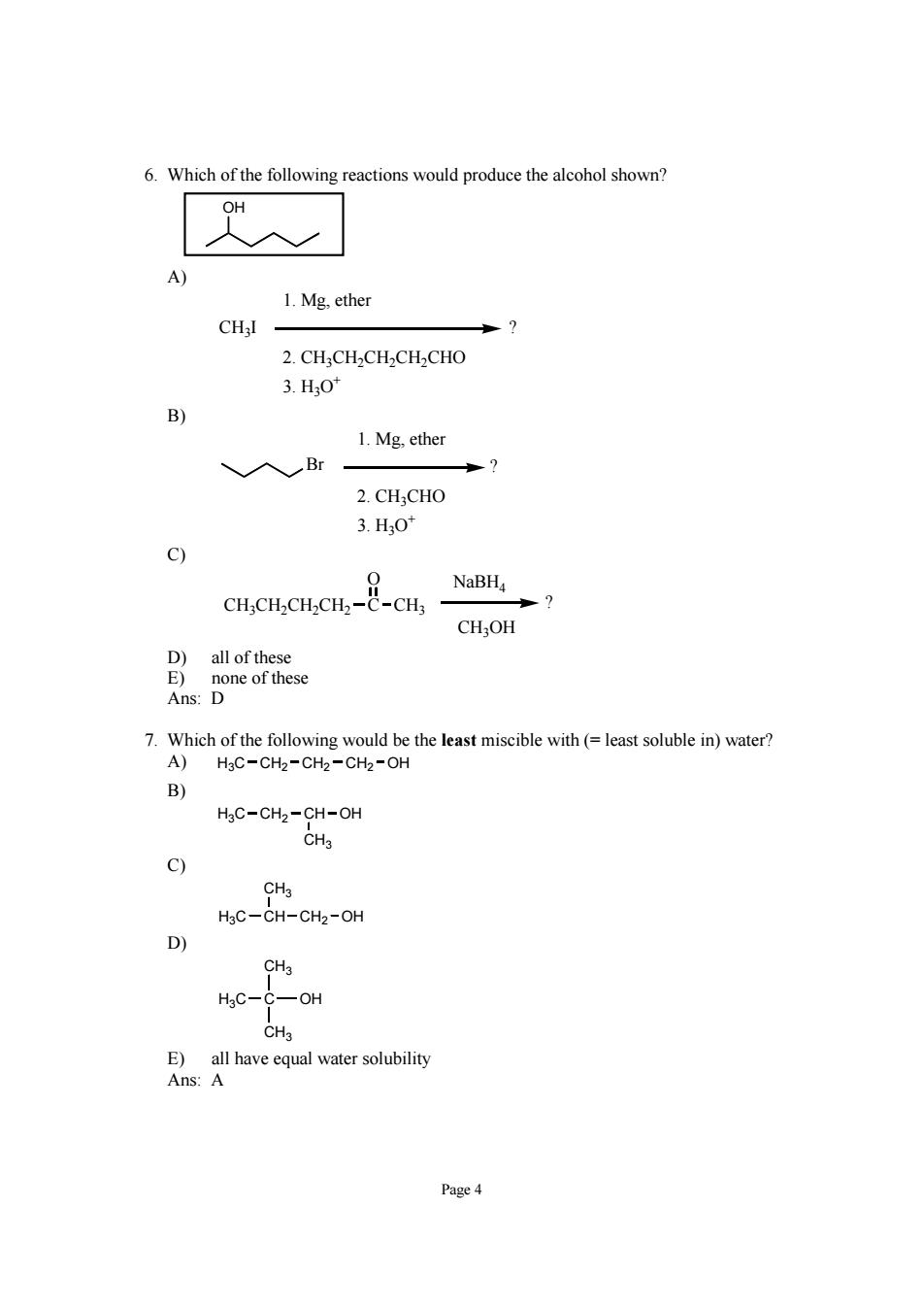
6.Which of the following reactions would produce the alcohol shown? 1.Mg,ether 2.CH:CHCHCH CHO 3.H0 B) 1.Mg,ether 、入Br 2.CH;CHO 3.H,0 9 NaBH p7 CH;OH mre re Ans:D 7.Which of the following would be the least miscible with(=least soluble in)water? A)H3C-CH2-CH2-CH2-OH B) C--o C) HsC-CH-CH2-OH D) CH3 HgC-C-OH Page4
Page 4 6. Which of the following reactions would produce the alcohol shown? OH A) CH3I 1. Mg, ether 2. CH3CH2CH2CH2CHO 3. H3O+ ? B) Br ? 1. Mg, ether 2. CH3CHO 3. H3O+ C) CH3CH2CH2CH2 C O CH3 NaBH4 CH3OH ? D) all of these E) none of these Ans: D 7. Which of the following would be the least miscible with (= least soluble in) water? A) H3C CH2 CH2 CH2 OH B) CH3 H3C CH2 CH OH C) CH3 H3C CH CH2 OH D) CH3 CH3 H3C C OH E) all have equal water solubility Ans: A
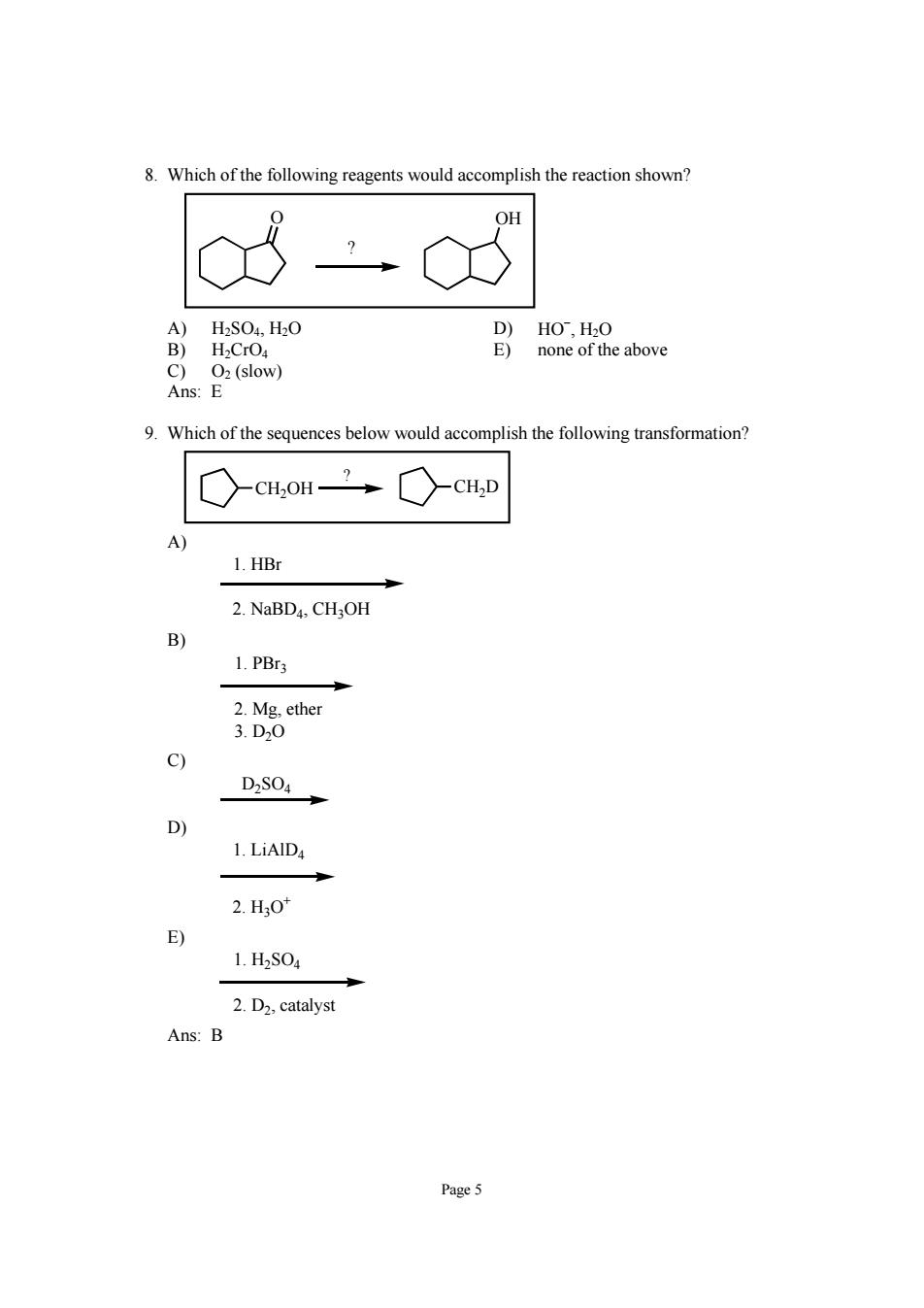
8.Which of the following reagents would accomplish the reaction shown? 8 OH A)H2S04,H0 B)H.CrO c Oz(slow) Ans:E 9.Which of the sequences below would accomplish the following transformation? ○cH,OH”○cH,D 1.HBr 2.NaBD4,CHOH B 1.PB3 2.Mg,ether 3.D0 C) D2S04 D) 2.H0t 1.H2S04 2.D2,catalyst Ans:B
Page 5 8. Which of the following reagents would accomplish the reaction shown? O OH ? A) H2SO4, H2O D) HO− , H2O B) H2CrO4 E) none of the above C) O2 (slow) Ans: E 9. Which of the sequences below would accomplish the following transformation? CH2OH CH2D ? A) 1. HBr 2. NaBD4, CH3OH B) 1. PBr3 2. Mg, ether 3. D2O C) D2SO4 D) 1. LiAlD4 2. H3O+ E) 1. H2SO4 2. D2, catalyst Ans: B