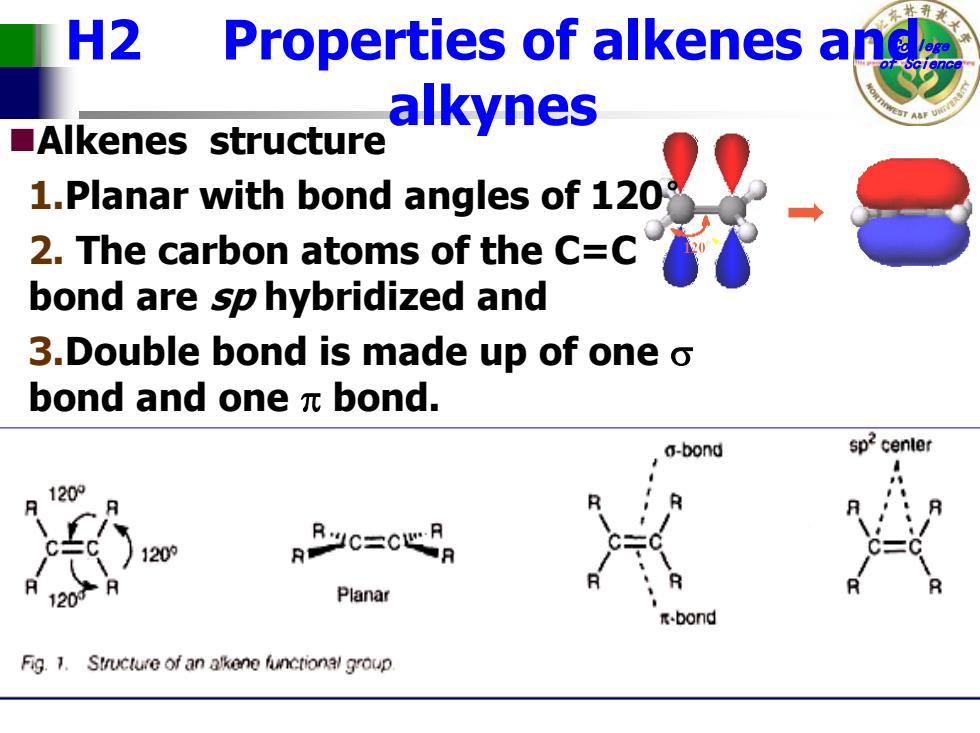
H2 Properties of alkenes and alkynes ■Alkenes structure 1.Planar with bond angles of 120 2.The carbon atoms of the C=C bond are sp hybridized and 3.Double bond is made up of one o bond and one元bond. a-bond 120° 120 120 Planar -bond Fig.1.Structure of an alkene functional group
College H2 Properties of alkenes andof Science alkynes Alkenes structure 1.Planar with bond angles of 120 ° 2. The carbon atoms of the C=C bond are sp hybridized and 3.Double bond is made up of one σ bond and one π bond

H2 Properties of alkenes and C=C alkynes Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present
College H2 Properties of alkenes andof Science C=C alkynes zBond rotation round a C=C bond is not possible zIsomers are possible depending on the substituents present
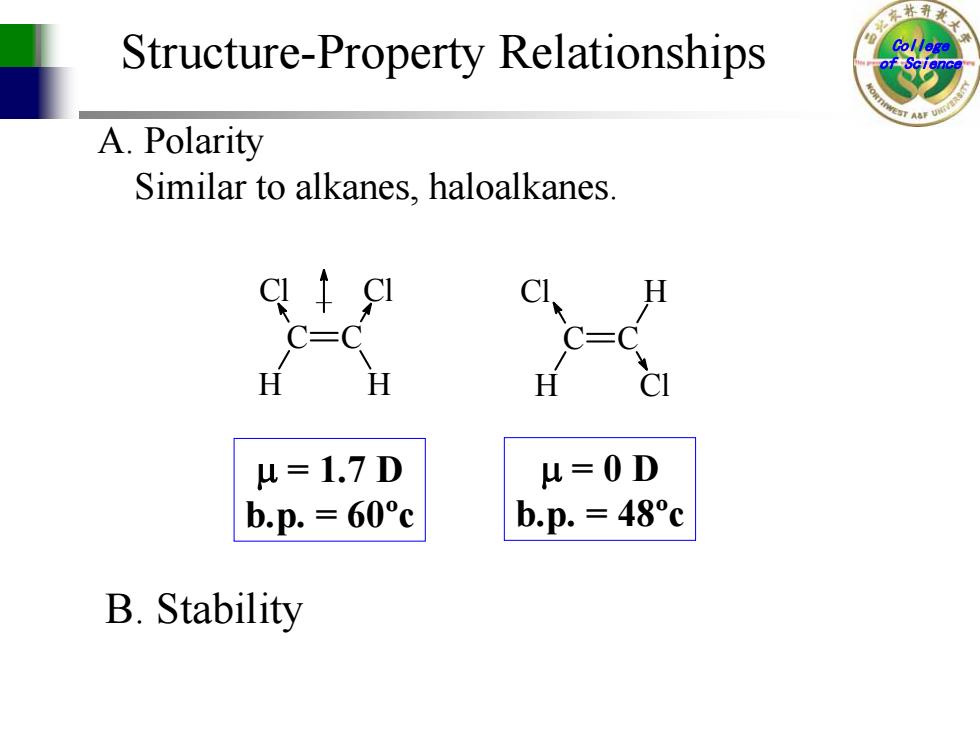
Structure-Property Relationships A.Polarity Similar to alkanes,haloalkanes. =1.7D 4=0D b.p.=60°c b.p.=48°c B.Stability
College Structure-Property Relationships of Science Similar to alkanes, haloalkanes. A. Polarity Cl C H C H Cl Cl C H C Cl H µ = 0 D b.p. = 48ºc µ = 1.7 D b.p. = 60ºc B. Stability

林 B.Stability Co I.more substituted more stable R R C=C C=( RCH=CHR,R2C=CH2 RCH-CH2>H2C=CH2 R The more substituents which are present on an alkene, the more stable the alkene is. 2.trans more stable than cis H H HH steric repulsion
College B. Stability of Science 1. more substituted = more stable R C R C R R R C R C H R > > RCH CHR , R2C CH2 > RCH CH2 > H2C CH2 2. trans more stable than cis C C H H H H H H H H C C H H H H H H H H The more substituents which are present on an alkene, the more stable the alkene is. steric repulsion
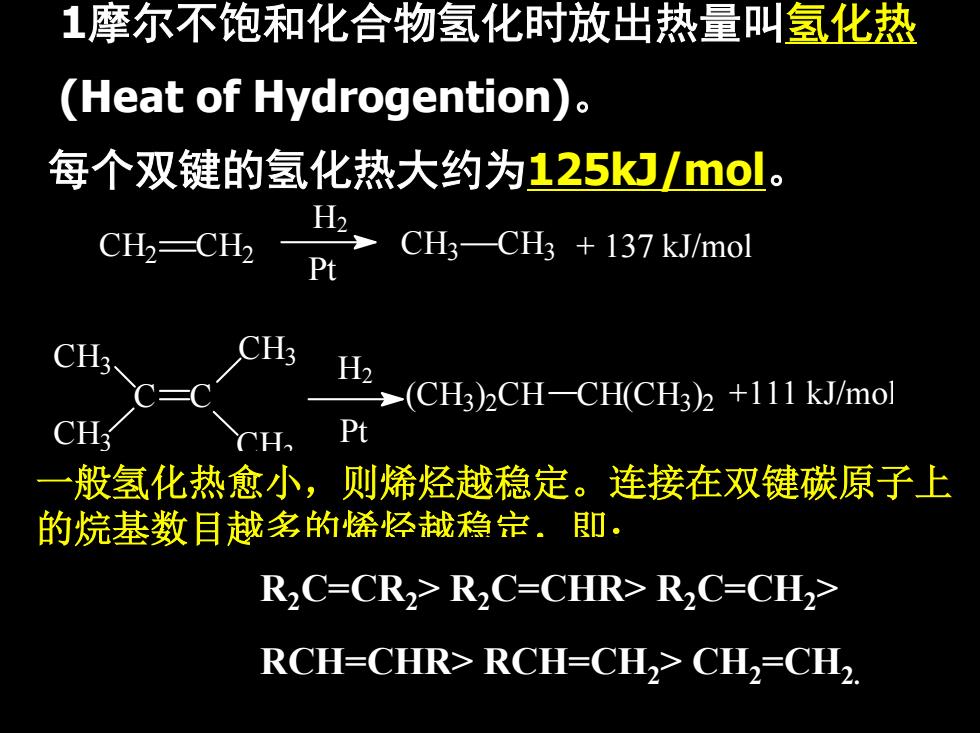
1摩尔不饱和化合物氢化时放出热量叫氢化热 (Heat of Hydrogention). 每个双键的氢化热大约为125kJ/mol。 CH2-CH2 H2 CHa-CH +137kJ/mol Pt H2 (CH3)CH-CH(CH3)2+111 kJ/mol CH Pt 一 般氢化热愈小,则烯烃越稳定。连接在双键碳原子上 的烷基数目越多的烯经战稳定.即: R2C=CR2>R2C=CHR>R2C=CH2> RCH=CHR>RCH=CH2>CH2=CH2
1摩尔不饱和化合物氢化时放出热量叫氢化热 (Heat of Hydrogention)。 每个双键的氢化热大约为125kJ/mol。 (CH +111 kJ/mol 3)2CH CH(CH3)2 H2 Pt C C CH3 CH3 CH3 CH3 CH3 CH3 + 137 kJ/mol Pt H2 CH2 CH2 一般氢化热愈小,则烯烃越稳定。连接在双键碳原子上 的烷基数目越多的烯烃越稳定,即: R2C=CR2> R2C=CHR> R2C=CH2> RCH=CHR> RCH=CH2> CH2=CH2