
Theoretical capacity At the cathode: LiFePO-Li+-xe FePO(@3-4 V vs.Li/Li+) Molecular weight of LiFePO: 6.94+55.85+31+16×4=157.79g/mol So,the theoretical capacity is: 96500÷157.79÷3.6=170 mA.h/g 6
Theoretical capacity At the cathode: LiFePO4 - Li+ - xe- ↔ FePO4 (@ 3 – 4 V vs. Li/Li+) Molecular weight of LiFePO4 : 6.94 + 55.85 + 31+16 × 4 = 157.79 g/mol So, the theoretical capacity is: 96500÷ 157.79 ÷3.6 = 170 mA·h/g 6
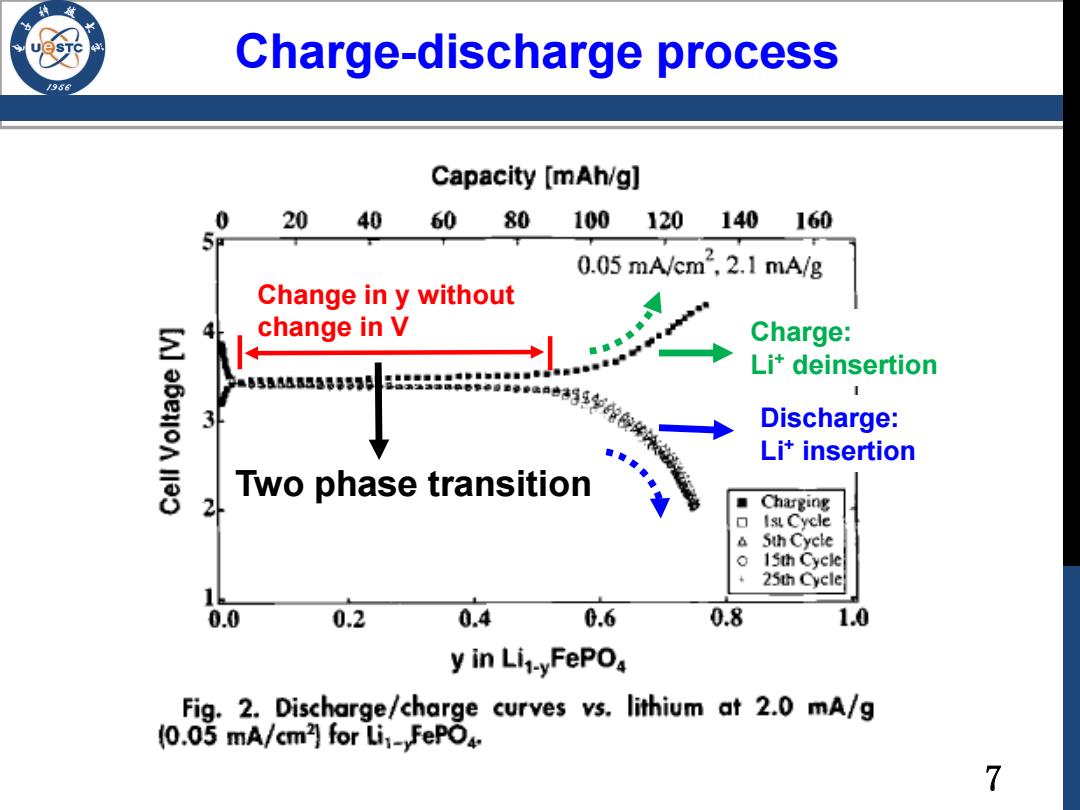
Charge-discharge process /986 Capacity [mAh/g] 0 2040 60 80 100 120 140 160 0.05mA/cm2,2.1mA/g Change in y without change in V Charge: Li*deinsertion 555188538553月 3 p靠年单e●a口的生学校共济、 Discharge: Lit insertion Two phase transition 2 Charging Ist Cycle Sth Cycle 0 15th Cycle 25th Cycle 0.0 0.2 0.4 0.6 0.8 1.0 y in Lit-yFePO Fig.2.Discharge/charge curves vs.lithium at 2.0 mA/g (0.05 mA/cm]for Li:-FePO4. 7
Charge-discharge process Change in y without change in V Discharge: Li+ insertion Charge: Li+ deinsertion Two phase transition 7
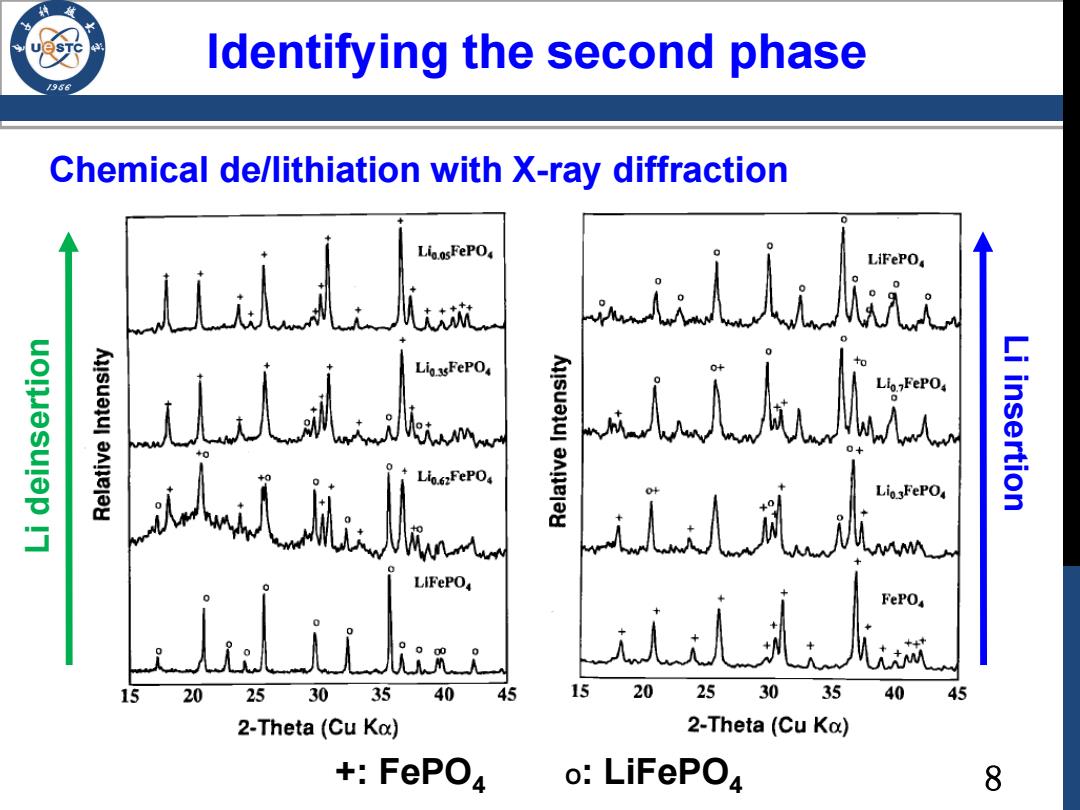
ldentifying the second l phase Chemical de/lithiation with X-ray diffraction Lio.osFePO4 人 LiFePO uolesulep !7 Lio.62FePO Li insertion Lio3FePO4 wM LIFePO i乱k 15 20 25 30 35 40 45 20 25 30 35 40 45 2-Theta(Cu Ko) 2-Theta(Cu Ka) +FePO4 o:LiFePO4 8
Chemical de/lithiation with X-ray diffraction Identifying the second phase Li deinsertion Li insertion +: FePO4 O: LiFePO4 8