
THE MOST WIDELY USED SURROGATE ENDPOINT IN DRUG DEVELOPMENT* Blood drug level as a surrogate endpoint for clinical efficacy and toxicity in the evaluation of generic drugs. Similar But Bioequivalence Ganand NottheSame 16
THE MOST WIDELY USED SURROGATE ENDPOINT IN DRUG DEVELOPMENT* Blood drug level as a surrogate endpoint for clinical efficacy and toxicity in the evaluation of generic drugs. 16 Bioequivalence

ONLY TWO SURROGATE ENDPOINTS FOR CARDIOVASCULAR DISEASE DRUGS* "The only surrogate endpoints currently used as a basis for approval of cardiovascular drugs are blood pressure and serum cholesterol level" Temple R:Are surrogate markers adequate to assess cardiovascular disease drugs?JAMA 1999;282:790-95. 17
ONLY TWO SURROGATE ENDPOINTS FOR CARDIOVASCULAR DISEASE DRUGS* “The only surrogate endpoints currently used as a basis for approval of cardiovascular drugs are blood 17 pressure and serum cholesterol level” * Temple R: Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 1999;282:790-95
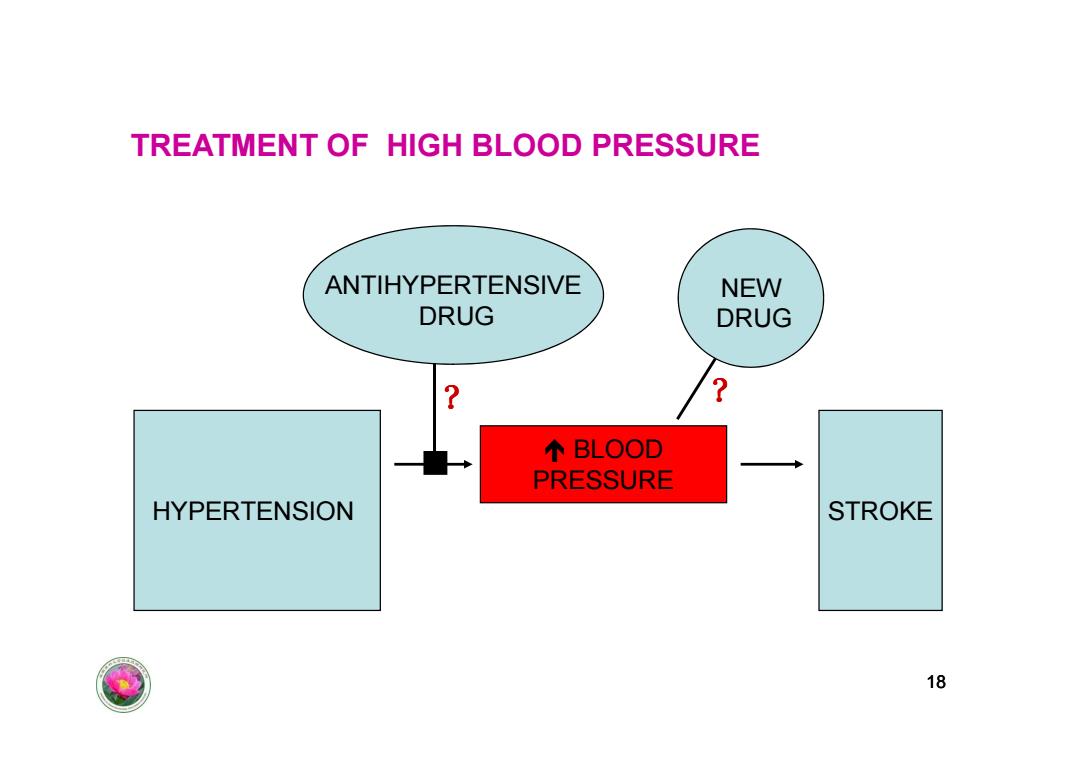
TREATMENT OF HIGH BLOOD PRESSURE ANTIHYPERTENSIVE NEW DRUG DRUG 个BLOOD PRESSURE HYPERTENSION STROKE 18
TREATMENT OF HIGH BLOOD PRESSURE NEW DRUG ? ANTIHYPERTENSIVE DRUG ? 18 HYPERTENSION STROKE BLOOD PRESSURE ? ?��

CHOLESTEROL CASE HISTORY Initial clinical utility as an epidemiologic biomarker Progression from biomarker to surrogate endpoint for drug developement:simvastatin SIMVASTATIN 40 uct Licence ho nPharm? 19
CHOLESTEROL CASE HISTORY • Initial clinical utility as an epidemiologic biomarker • Progression from biomarker to surrogate endpoint for drug developement: simvastatin 19
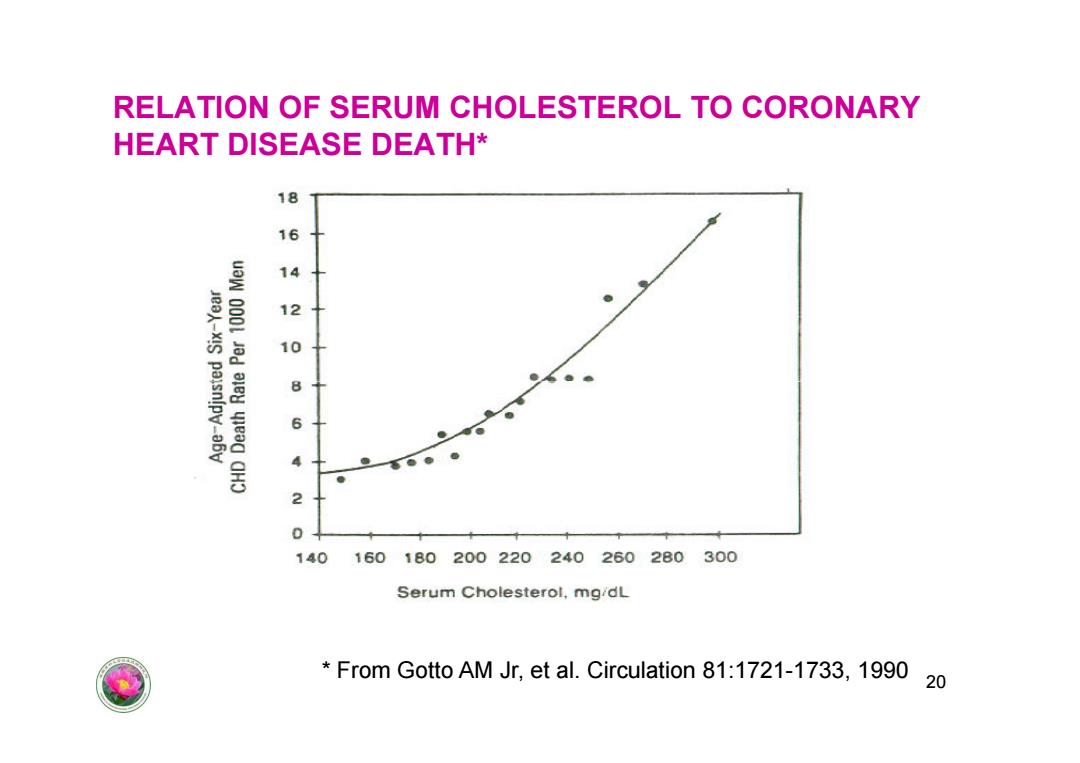
RELATION OF SERUM CHOLESTEROL TO CORONARY HEART DISEASE DEATH* 18 16 14 12 Je-xIS paisn!py-a6v 10 8 6 2 140 160180200220240260280300 Serum Cholesterol,mg/dL From Gotto AM Jr,et al.Circulation 81:1721-1733,1990 20
RELATION OF SERUM CHOLESTEROL TO CORONARY HEART DISEASE DEATH* 20 * From Gotto AM Jr, et al. Circulation 81:1721-1733, 1990