
I2.Preparation and properties of Aromatic compounds Physical Properties of benzene 1.It is a colourless liquid with boiling point 80C. 2.It is insoluble in water but soluble in organic solvent. 3.It burns with a sooty flame and it is highly inflammable. 4.It is carcinogenic.致癌物(质)的
College of Science I2、Preparation and properties of Aromatic compounds Physical Properties of benzene 1. It is a colourless liquid with boiling point 80oC. 2. It is insoluble in water but soluble in organic solvent. 3. It burns with a sooty flame and it is highly inflammable. 4. It is carcinogenic.致癌物(质)的
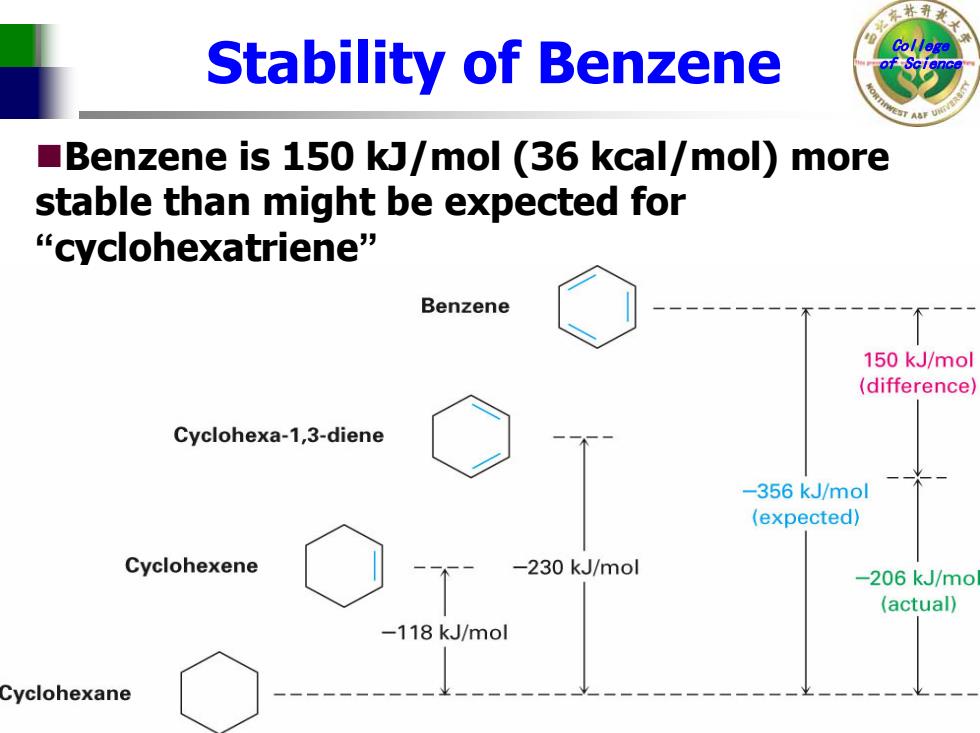
Stability of Benzene Benzene is 150 kJ/mol (36 kcal/mol)more stable than might be expected for “cyclohexatriene” Benzene 150 kJ/mol (difference) Cyclohexa-1,3-diene -356 kJ/mol (expected) Cyclohexene -230 kJ/mol -206 kJ/mo (actual) -118 kJ/mol Cyclohexane
College Stability of Benzene of Science Benzene is 150 kJ/mol (36 kcal/mol) more stable than might be expected for “cyclohexatriene

林 Chemical Properties of benzene Electrophilic Substitution Reaction a. Nitration (Forming nitro-compound) b.Sulphonation (Forming sulphonic acid) c.Halogenation (Forming halobenzene) d.Friedal-Craft Alkylation (Forming alkylbenzene) e. Friedal-Craft Acylation (Forming carbonyl compound)
College of Science Chemical Properties of benzene Electrophilic Substitution Reaction a. Nitration (Forming nitro-compound) b. Sulphonation (Forming sulphonic acid) c. Halogenation (Forming halobenzene) d. Friedal-Craft Alkylation (Forming alkylbenzene) e. Friedal-Craft Acylation (Forming carbonyl compound)

I2,Preparation and properties ol loge of Aromatic compounds ■Preparation Hal ●Simple aromatic structures such as benzene,toluene,or Halogenation Acylation naphthalene are isolated from natural NO2 sources and converted Aromatic ring to more complex Nitration Alkylation aromatic structures. SO3H OH Sulfonation Hydroxylation
College I2、Preparation and properties of Science of Aromatic compounds Preparation zSimple aromatic structures such as benzene, toluene, or naphthalene are isolated from natural sources and converted to more complex aromatic structures

12,Preparation and properties of Aromatic compounds ■Properties .Many aromatic compounds have a characteristic aroma and burn with a smoky flame. .They are nonpolar,hydrophobic molecules which dissolve in organic solvents rather than water. Aromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. Electrophilic substitution is the most common type of reaction. However,reduction is also possible. b)】 ---6+ van der Waals interactions Induced dipole interaction Fig.1.Intermolecular bonding involving aromatic rings
College of Science I2、 Preparation and properties of Aromatic compounds Properties zMany aromatic compounds have a characteristic aroma and burn with a smoky flame. zThey are nonpolar, hydrophobic molecules which dissolve in organic solvents rather than water. zAromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. zElectrophilic substitution is the most common type of reaction. However, reduction is also possible