
2013-3-6 Chapter 6 Using Entropy Learning Outcomes Demonstrate understanding of key concepts related to entropy and the second law...including entropy transfer, entropy production,and the increase in entropy principle. Evaluate entropy,evaluate entropy change between two states,and analyze isentropic processes,using appropriate property tables
2013-3-6 1 Chapter 6 Using Entropy Learning Outcomes ►Demonstrate understanding of key concepts related to entropy and the second law . . . including entropy transfer, entropy production, and the increase in entropy principle. ►Evaluate entropy, evaluate entropy change between two states, and analyze isentropic processes, using appropriate property tables

2013-3-6 Learning Outcomes,cont. Represent heat transfer in an internally reversible process as an area on a temperature-entropy diagram. Apply entropy balances to closed systems and control volumes. Evaluate isentropic efficiencies for turbines,nozzles,compressors,and pumps. Introducing Entropy Change and the Entropy Balance Mass and energy are familiar extensive properties of systems.Entropy is another important extensive property. Just as mass and energy are accounted for by mass and energy balances,entropy is accounted for by an entropy balance. Like mass and energy,entropy can be transferred across the system boundary. 2
2013-3-6 2 Learning Outcomes, cont. ►Represent heat transfer in an internally reversible process as an area on a temperature-entropy diagram. ►Apply entropy balances to closed systems and control volumes. ►Evaluate isentropic efficiencies for turbines, nozzles, compressors, and pumps. Introducing Entropy Change and the Entropy Balance ►Mass and energy are familiar extensive properties of systems. Entropy is another important extensive property. ►Just as mass and energy are accounted for by mass and energy balances, entropy is accounted for by an entropy balance. ►Like mass and energy, entropy can be transferred across the system boundary
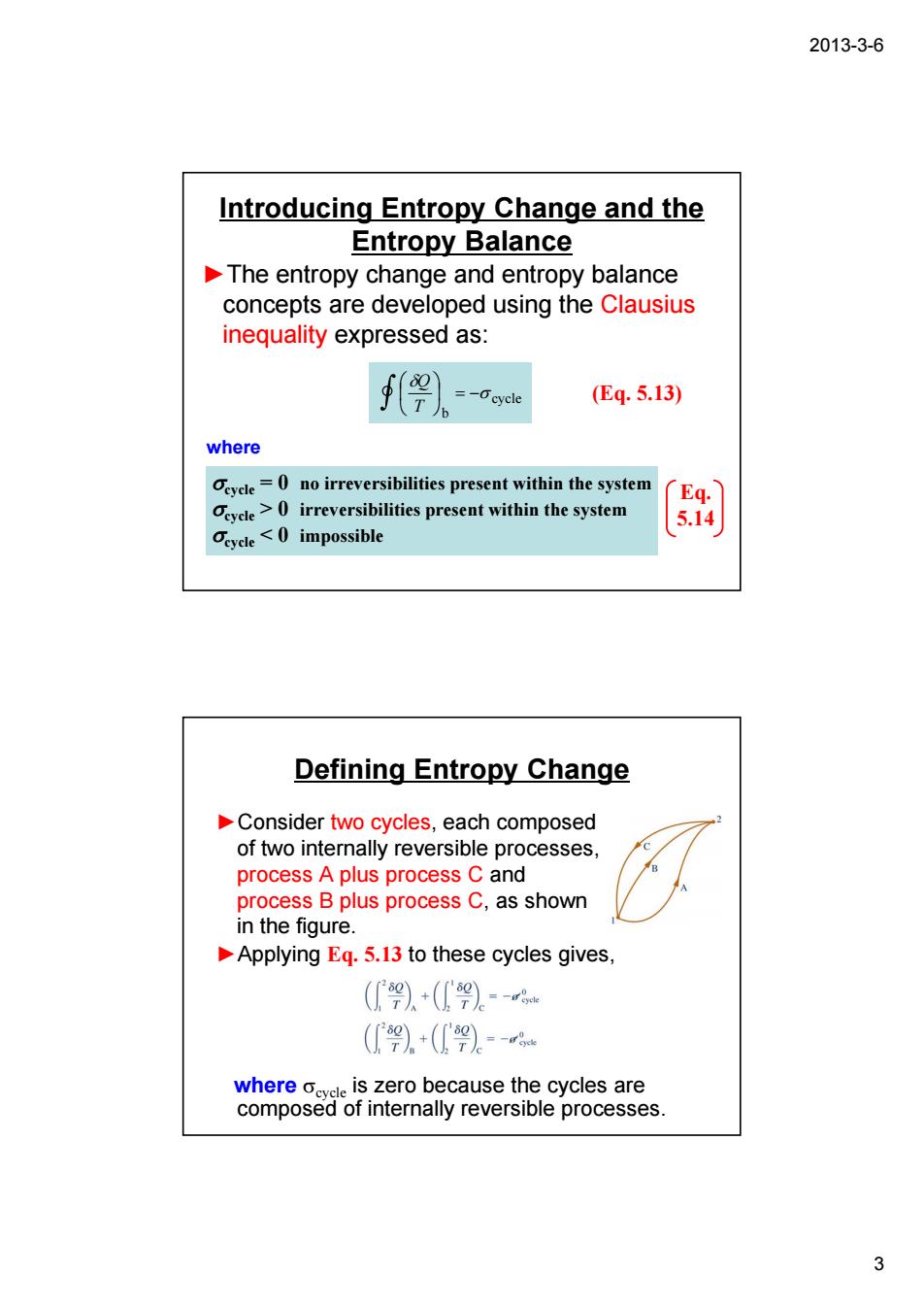
2013-3-6 Introducing Entropy Change and the Entropy Balance The entropy change and entropy balance concepts are developed using the Clausius inequality expressed as: f9)- (Eq.5.13) where irreversibilities present within the system Oevcle>0 irreversibilities present within the system eycle impossible Defining Entropy Change Consider two cycles,each composed of two interally reversible processes process A plus process C and process B plus process C,as shown in the figure. Applying Eq.5.13 to these cycles gives (.+(9)a (+(》。- tepRseao57Renbfraetenshie2c6eaes 3
2013-3-6 3 Introducing Entropy Change and the Entropy Balance ►The entropy change and entropy balance concepts are developed using the Clausius inequality expressed as: cycle b σ δ ⎟ = − ⎠ ⎞ ⎜ ⎝ ⎛ T Q (Eq. 5.13) where σcycle = 0 no irreversibilities present within the system σcycle > 0 irreversibilities present within the system σcycle < 0 impossible Eq. 5.14 Defining Entropy Change ►Consider two cycles, each composed of two internally reversible processes, process A plus process C and process B plus process C, as shown in the figure. ►Applying Eq. 5.13 to these cycles gives, where σcycle is zero because the cycles are composed of internally reversible processes

2013-3-6 Defining Entropy Change Subtracting these equations: Since A and B are arbitrary internally reversible processes linking states 1 and 2,it follows that the value of the integral is independent of the particular internally reversible process and depends on the end states only. Defining Entropy Change Recalling (from Sec.1.3.3)that a quantity is a property if. and only if,its change in value between two states is independent of the process linking the two states,we conclude that the integral represents the change in some property of the system. We call this represent it by The change in entropy is 5-5-(9) (Eq.6.2a) where the subscript "int rev"signals that the integral is carried out for any intemally reversible process linking states 1 and 2. 4
2013-3-6 4 Defining Entropy Change ►Subtracting these equations: ►Since A and B are arbitrary internally reversible processes linking states 1 and 2, it follows that the value of the integral is independent of the particular internally reversible process and depends on the end states only. Defining Entropy Change ►Recalling (from Sec. 1.3.3) that a quantity is a property if, and only if, its change in value between two states is independent of the process linking the two states, we conclude that the integral represents the change in some property of the system. ►We call this property entropy and represent it by S. The change in entropy is where the subscript “int rev” signals that the integral is carried out for any internally reversible process linking states 1 and 2. (Eq. 6.2a)

2013-3-6 Defining Entropy Change Equation 6.2a allows the change in entropy between two states to be determined by thinking of an internally reversible process between the two states.But since enropy isa property,that value ofenropy change applies to any process between the states-internally reversible or not. Entropy change is introduced by the integral of Eq.6.2 for which no accompanying physical picture is given.Still the aim of Chapter 6 is to demonstrate that entropy not only has physical significance but also is essential for thermodynamic analysis. Entropy Facts Entropy is an extensive property. Like any other extensive property,the change in entropy can be positive,negative,or zero: >0 S2-S:{=0 <0 By inspection of Eq.6.2a,units for entropy S are kJ/K and Btu/R. Units for specific entropy s are kJ/kg-K and Btu/Ib.R. 5
2013-3-6 5 Defining Entropy Change ►Equation 6.2a allows the change in entropy between two states to be determined by thinking of an internally reversible process between the two states. But since entropy is a property, that value of entropy change applies to any process between the states – internally reversible or not. ►Entropy change is introduced by the integral of Eq. 6.2a for which no accompanying physical picture is given. Still, the aim of Chapter 6 is to demonstrate that entropy not only has physical significance but also is essential for thermodynamic analysis. Entropy Facts ►Entropy is an extensive property. ►Like any other extensive property, the change in entropy can be positive, negative, or zero: ►By inspection of Eq. 6.2a, units for entropy S are kJ/K and Btu/oR. ►Units for specific entropy s are kJ/kg·K and Btu/lb· oR