
190 悠 、O入VIW1VE农、 Chapter 5.Phase diagrams ”大餐横城工置部 w SINO-ITALIAN CAMPUS
SINO-ITALIAN CAMPUS Chapter 5. Phase diagrams

190> Exercises 1.Define (a)phase in a material and (b)a phase diagram. 2.Write the equation for Gibbs phase rule and define each of the terms 3.What are the four Hume-rothery rules for the solid solubility of one element in another 4.Cite three variables that determine the microstructure of an alloy 5.What thermodynamic condition must be met for a state of equilibrium to exit 6.What is the difference between the states of phase equilibrium and metastability SINO-ITALIAN CAMPUS
SINO-ITALIAN CAMPUS Exercises 1. Define (a) phase in a material and (b) a phase diagram. 2. Write the equation for Gibbs phase rule and define each of the terms 3. What are the four Hume-rothery rules for the solid solubility of one element in another 4. Cite three variables that determine the microstructure of an alloy 5. What thermodynamic condition must be met for a state of equilibrium to exit 6. What is the difference between the states of phase equilibrium and metastability
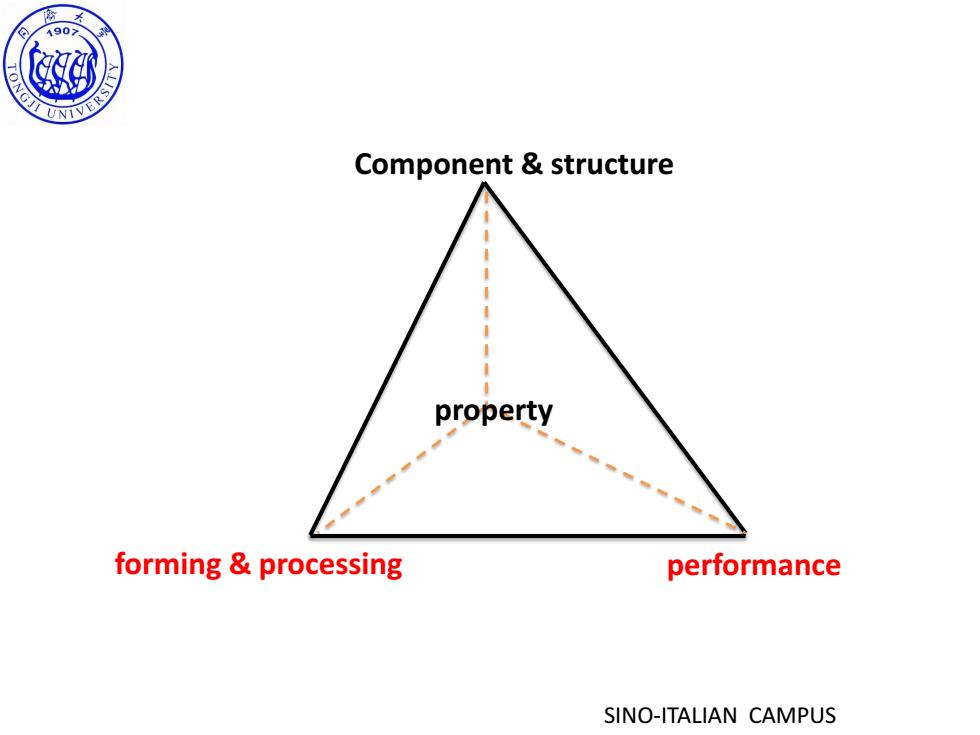
190习 TONGJI UNIVER、 Component structure property forming processing performance SINO-ITALIAN CAMPUS
SINO-ITALIAN CAMPUS Component & structure forming & processing performance property
190> OUTLINE 1.Definition and basic concepts 2.The interpretation of diagram 3.The study of a development phase diagram 4.Study of a binary alloy phase diagram SINO-ITALIAN CAMPUS
SINO-ITALIAN CAMPUS OUTLINE 1. Definition and basic concepts 2. The interpretation of diagram 3. The study of a development phase diagram 4. Study of a binary alloy phase diagram

190> 5.1 Definitions Components:pure metals and/or compounds of which an alloy is composed. "system":a specific body of material under consideration.Or it may relate to the series of possible alloys consisting of the same compounds,but without regard to alloy composition. "Solvent"represents the element or compound that in the greatest amount(host atoms,host material). "Solute"is used to denote an element or compound present in a minor concentration. Solid solution SINO-ITALIAN CAMPUS
SINO-ITALIAN CAMPUS 5.1 Definitions Components: pure metals and/or compounds of which an alloy is composed. “system” : a specific body of material under consideration. Or it may relate to the series of possible alloys consisting of the same compounds, but without regard to alloy composition. “Solvent” represents the element or compound that in the greatest amount( host atoms, host material). ”Solute” is used to denote an element or compound present in a minor concentration. Solid solution