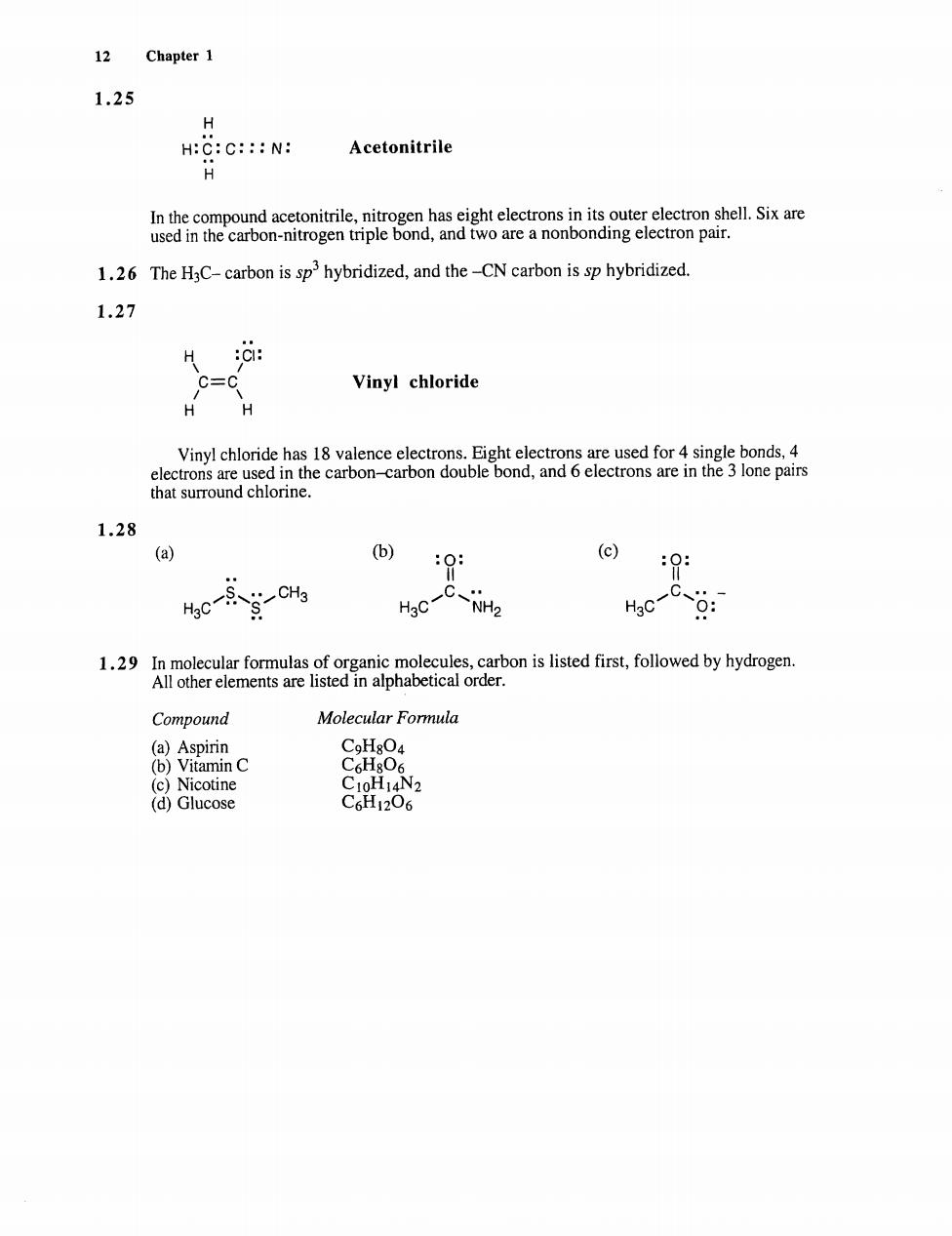
12 Chapter 1 1.25 H:C:C:::N: Acetonitrile 1.26 The HaC-carbon is sp3hybridized,and the-CN carbon is sp hybridized. 1.27 H :C: C= Vinyl chloride H H Vinyl chloride has 18 valence electrons.Eight electrons are used for 4 single bonds,4 electrons are used in the carbon-carbon double bond,and 6 electrons are in the 3 lone pairs that surround chlorine. 1.28 (a) (b) 0 1.29 In molecular formulas of organic molecules,carbon is listed first,followed by hydrogen. All other elements are listed in alphabetical order. Compound Molecular Formula (a)Aspirin CoHg04 (b)Vitamin C 61806
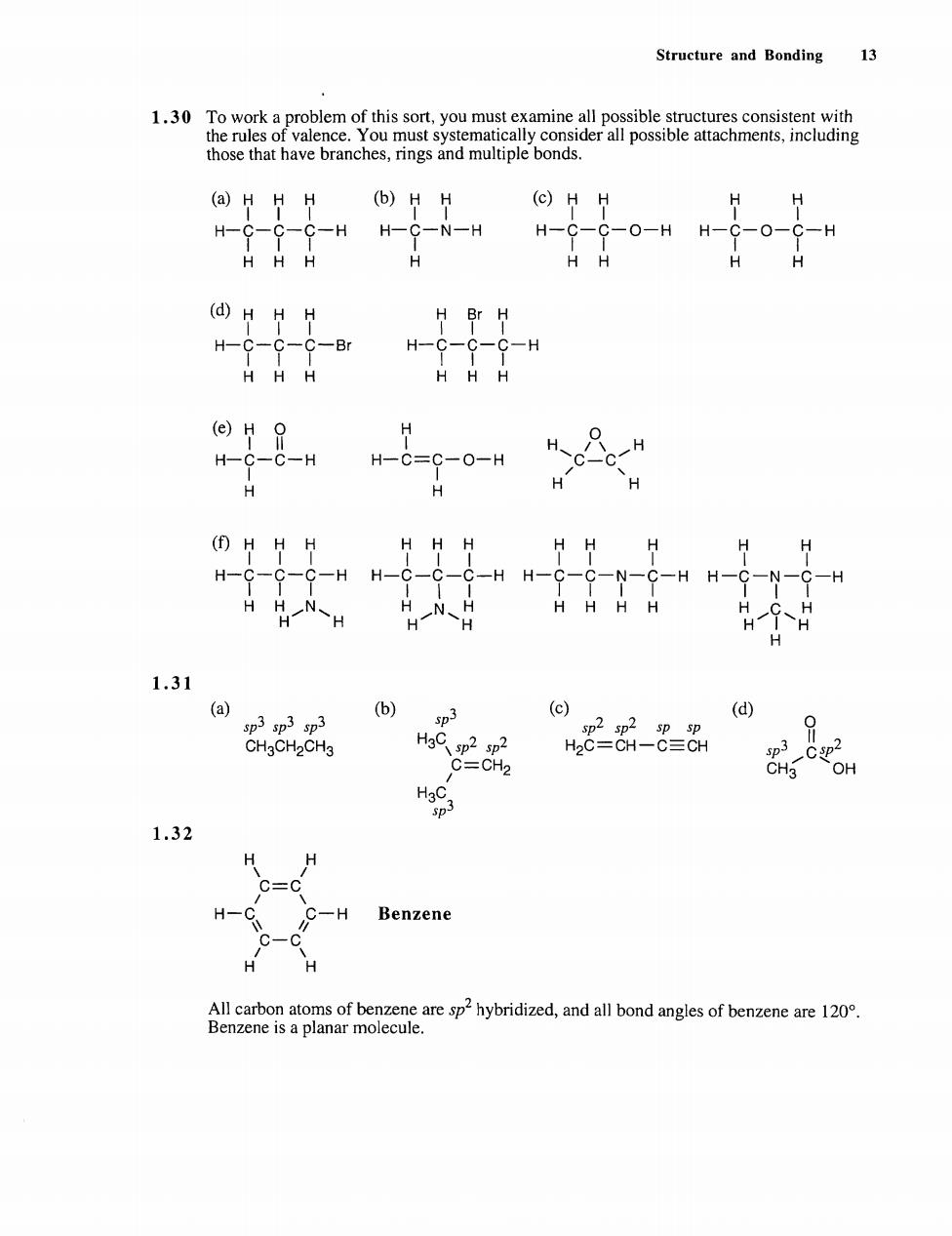
Structure and Bonding 13 e vY ymynee m those that have branches,rings and multiple bonds. (a)HHH (6)HH (C)HH H-C-C-C-H H- -N-H H-C- --0-HH-G-0--H HHH H H (d)HHH H Br H H-C-C-C-Br H-C-C-C-H HHH HHH (e)H H H-C-C-H H-C=C-0-H H H H 田HHH H HH HHH H H--9-9-HH- -H H-C-C-N-C -H H-C HH.NH HN H HHHH 1.31 () (d) HaC sp2 sp2 82-8 C=CH2 1.32 H -H Benzene H All carbon atoms of benzene are sp2hybridized,and all bond angles of benzene are 120 Benzene is a planar molecule
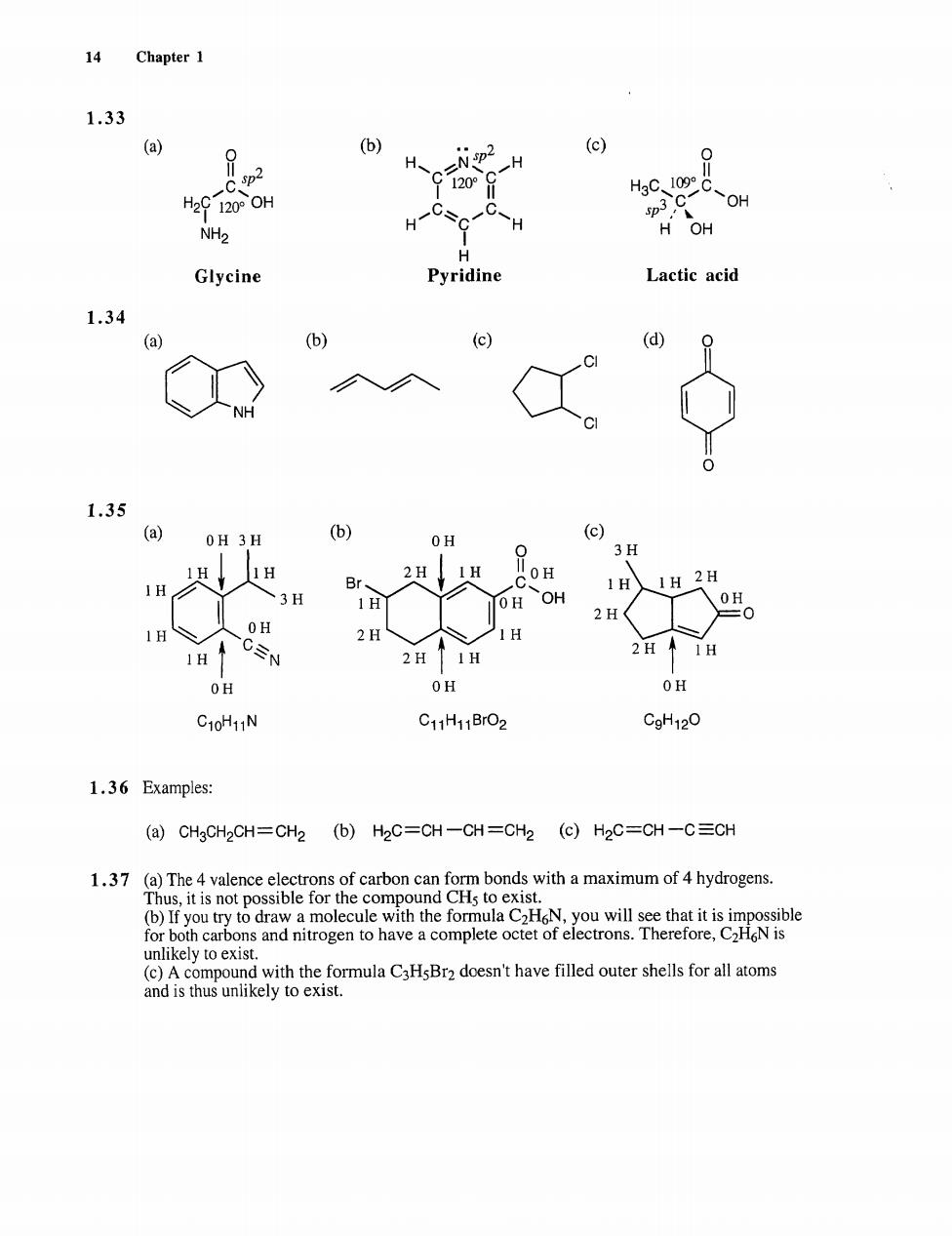
g Chapter 1 1.33 (a) P (b) (c) 2 0 1209 y 人 NH2 Glycine Pyridine Lactic acid 1.34 (a) (c) (d) 1.35 (a) OH 3H (b) OH (c) 3H H OH OH H OH C10H11N CgH12O 1.36 Examples: (a)CHgCH2CH=CH2 (b)H2C=CH-CH=CH2 (c)H2C=CH-C=CH 1.37 (a)The onds with a maximum of 4 hydrogens. (b)If you t tomocuth th formulaNyou will se thatt orbooadnrogen t havecomptocteoftron.ThereforeN unlikely to exist. and is tus to e
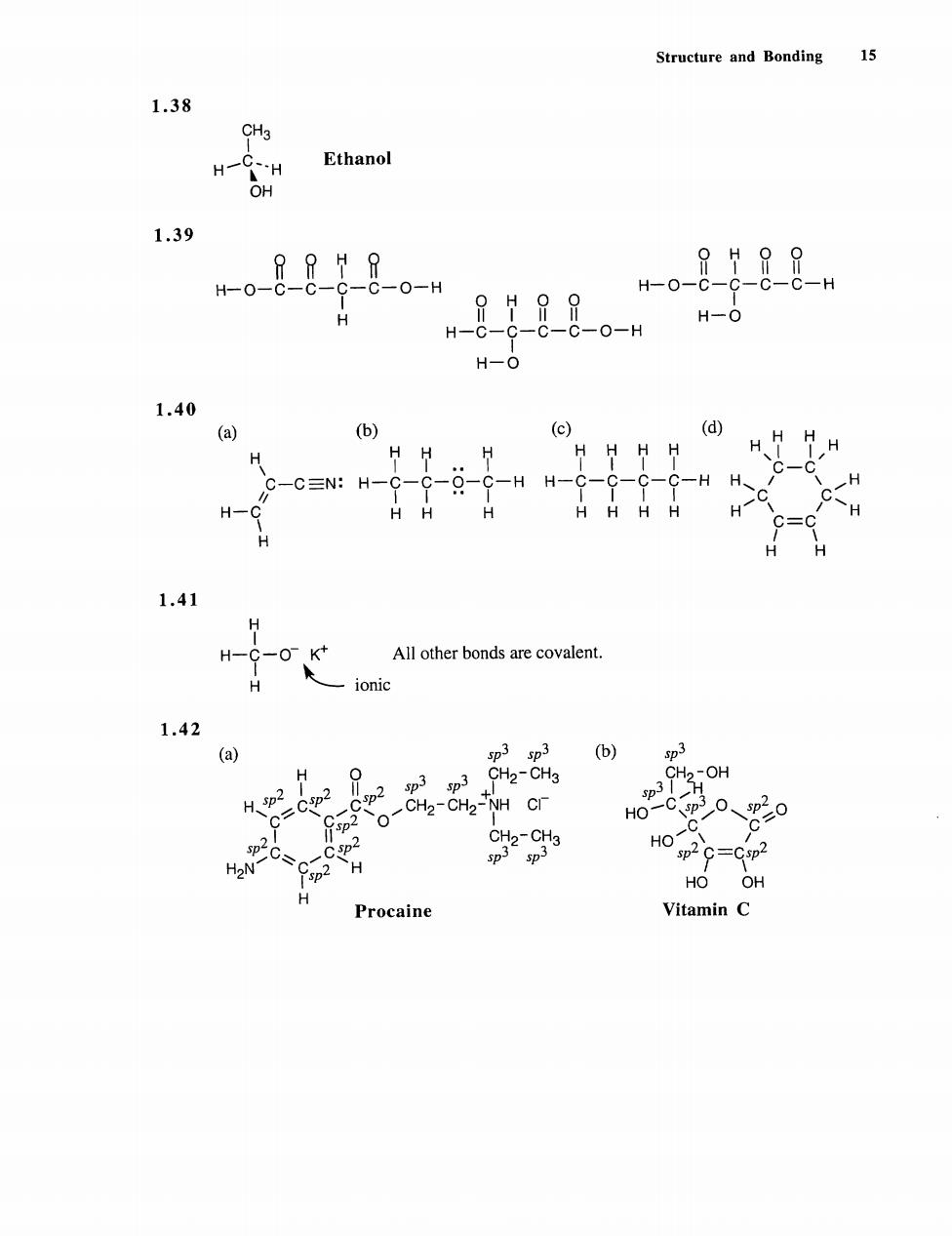
Structure and Bonding 15 1.38 CH3 Ethanol 1.39 o-8-8-8-8。-n 88-8- H-0- 8g-8-8。-” H-0 1.40 (a) (b) (c) (d) HHHHHHH HH H H -c:H-9-9-g-9-HH-9-9--9-H8 H-C HHH HHHHH 1.41 H-C-0 K All other bonds are covalent. -ionic 1.42 o sp3 sp3 (b) 0 p2p3 sp3 CH2-CH3 CH2-CH2-NH CI H0-cP0、o H2N sp- HO OH Procaine Vitamin C
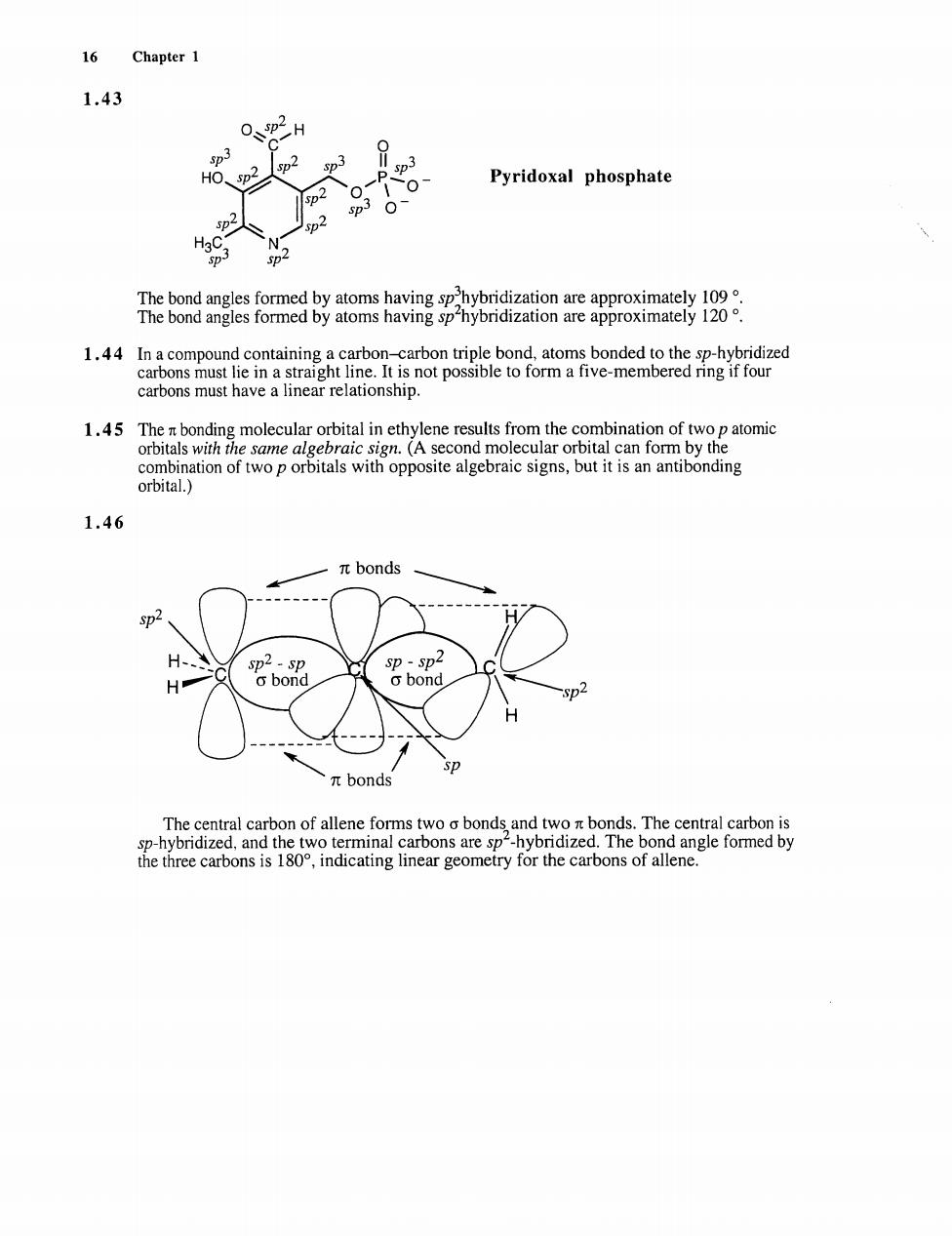
16 Chapter 1 1.43 0、p2H p Pyridoxal phosphate 1.44【n a compou carbons must have a linear relationship. 0 1.46 πbonds H o品 sp πbonds the three carbons is 180,indicating linear geometry for the carbons of allene