
Structure and Bonding 7 1.8 rhand d ydrogslleronsre onded tofour H片H -C-C-H Propane HHH The geometry around all carbon atoms is tetrahedral,and all bond angles are approximately 1099 1.9 H、 H-C-C Hexane HH HH HH 1.10 32 Propene p2 H orbi TeHdCI-H bonds are bonds formed by overlap of an sp2orbital of carbon with an s orbital of hydrogen. The C2-C3 bond is a o bond formed by overlap of an sp'orbital of carbon 3 with an sp2orbital of carbon 2. There are two C1-C2 bonds.One is aobond formed by overlap of an sp2orbital of carbon 1 with an sp2orbital of carbon 2.The other is a n bond formed by overlap of a p orbital of carbon I carbor biCaRa9ieialotcartan2nAifouraomsconecic8Hhe are nydrogen a 1.11 H and all bond all atoms lie in the sar sp2 H H 1,3-Butadiene
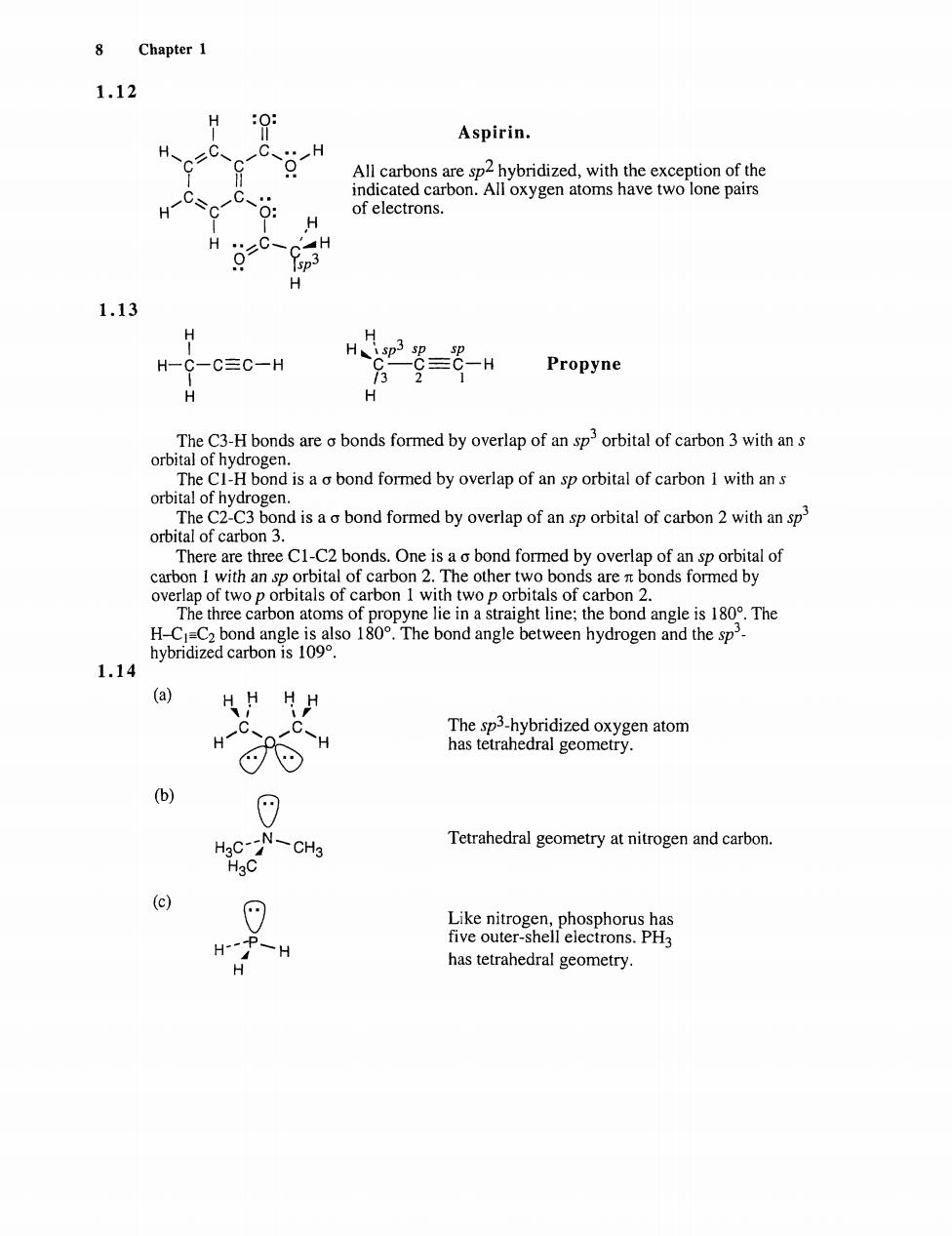
8 Chapter 1 1.12 Aspirin. All carbons are sp2 hybridized,with the exception of the indicated carbon.All oxygen atoms have two lone pairs of electrons. H H p H 1.13 H-C-C≡C-H Propyne The C3-H bonds are a bonds formed by overlap of an sporbital of carbon 3 with an s ebod formed by overlp of orbtlof caon orbital of carbon 3. There are three CI-C2 bonds.One is a bond formed by overlap of an sp orbital of lof carbon 2. The other t rbo ns of e li nd ngle is180.The H.CC nd an en hydrogen nd the sp hybridized carbon is 109. 1.14 (a) HH片H H-C C-H The sp3-hybridized oxygen atom has tetrahedral geometry. CN-a Tetrahedral geometry at nitrogen and carbon. Like ni W-H has tetrahedral geometry

Structure and Bonding 9 (d) H3C、」 CH2CH2CHCOH ©NH2 owhIhydrogen and a four-way inersection presentscarbonh Solution: (a) OH 1H 6 3H HO NHCH3 1H 2H OH 2H IH >0H 2H Adrenaline-CgH13NO3 2H OH Estrone-C18H2202 1.16 Several possible skeletal structures can satisfy each molecular formula @c2 (b) C2HㄣN NH、 (e) C3H50 入0 H 8 OH 、则 (d) 人

10 Chapter 1 1.17 PABA Visualizing Chemistry 1.18 片H H H H CgH17N H NH2 H:N-H 0-4 H-( -G-9-H HH :O: C3HzNO2 1.19 Citric acid (C6HgO7)contains seve s,each of which has two electron lone H、 0:H 980:9 -8 -H Citric acid

Structure and Bonding 1.20 1.2 Aspartame Additional Problems 1.22 Atomic Number of Element Number valence electrons (a)Zin (b)Iodine 39 2 岛o 2站 4 2(in 4s subshell).6(in 3d subshell) 1.23 Ground-state Element electron configuration (c)Aluminum 13 (d)Germanium 2 1.24(a)NH20H (b)AICl3 (c)CF2Cl2 (d)CH2O