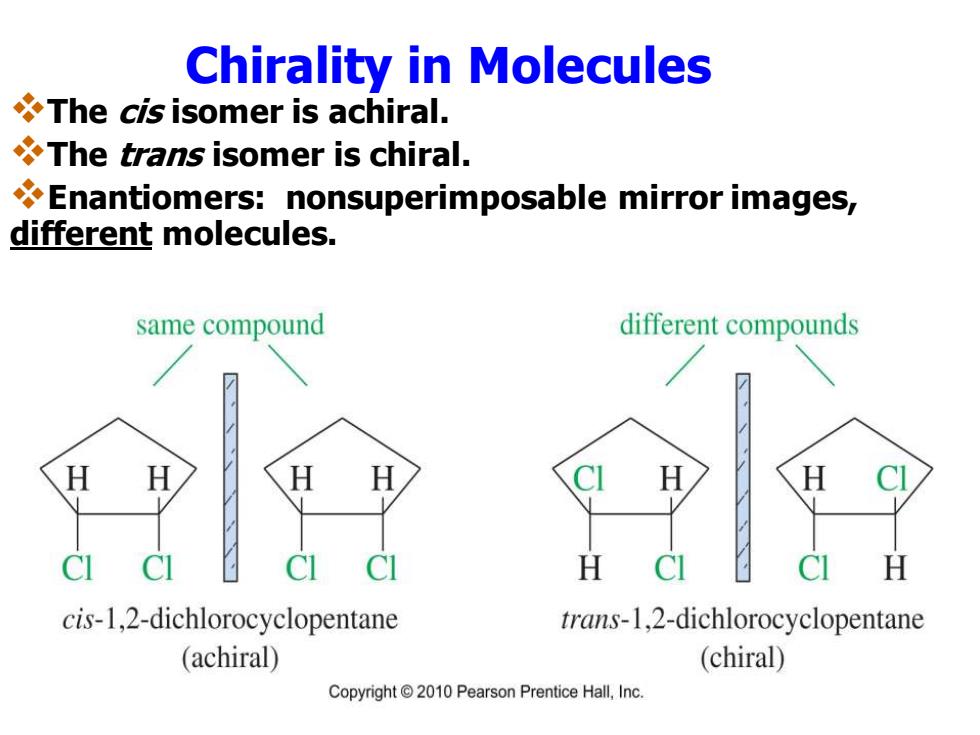
Chirality in Molecules The cis isomer is achiral. The trans isomer is chiral. Enantiomers:nonsuperimposable mirror images, different molecules. same compound different compounds H cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane (achiral) (chiral) Copyright 2010 Pearson Prentice Hall,Inc
Chirality in Molecules ❖The cis isomer is achiral. ❖The trans isomer is chiral. ❖Enantiomers: nonsuperimposable mirror images, different molecules
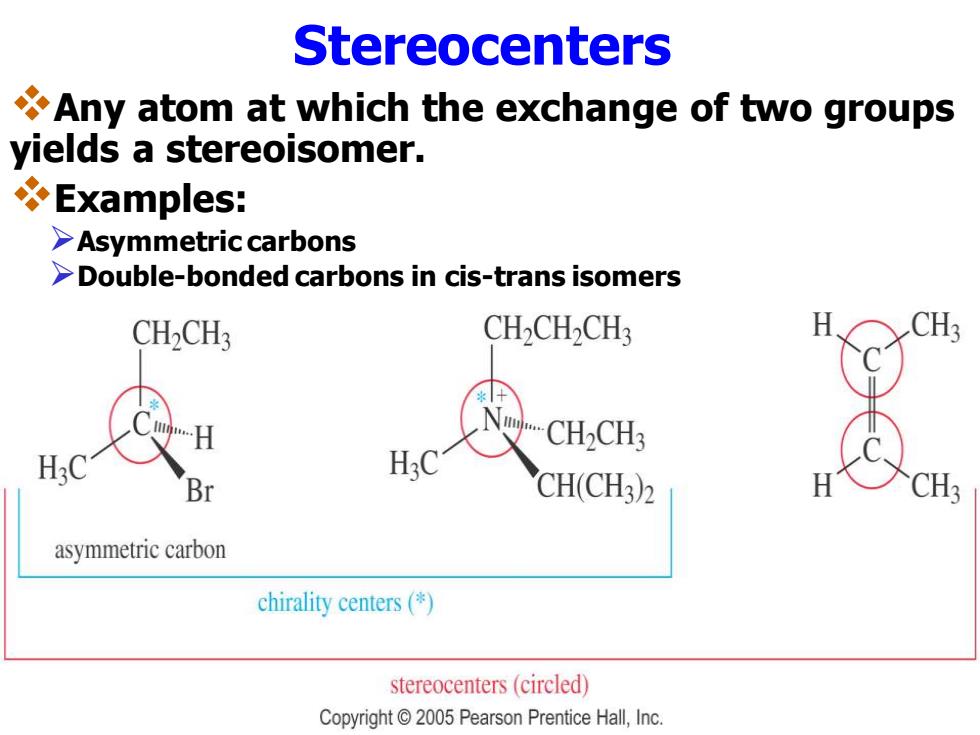
Stereocenters Any atom at which the exchange of two groups yields a stereoisomer. Examples: >Asymmetric carbons >Double-bonded carbons in cis-trans isomers CH>CH3 CH2CH2CH3 CH2CH3 H30 Br CH(CH3)2 CH3 asymmetric carbon chirality centers() stereocenters(circled) Copyright 2005 Pearson Prentice Hall,Inc
Stereocenters ❖Any atom at which the exchange of two groups yields a stereoisomer. ❖Examples: ➢Asymmetric carbons ➢Double-bonded carbons in cis-trans isomers
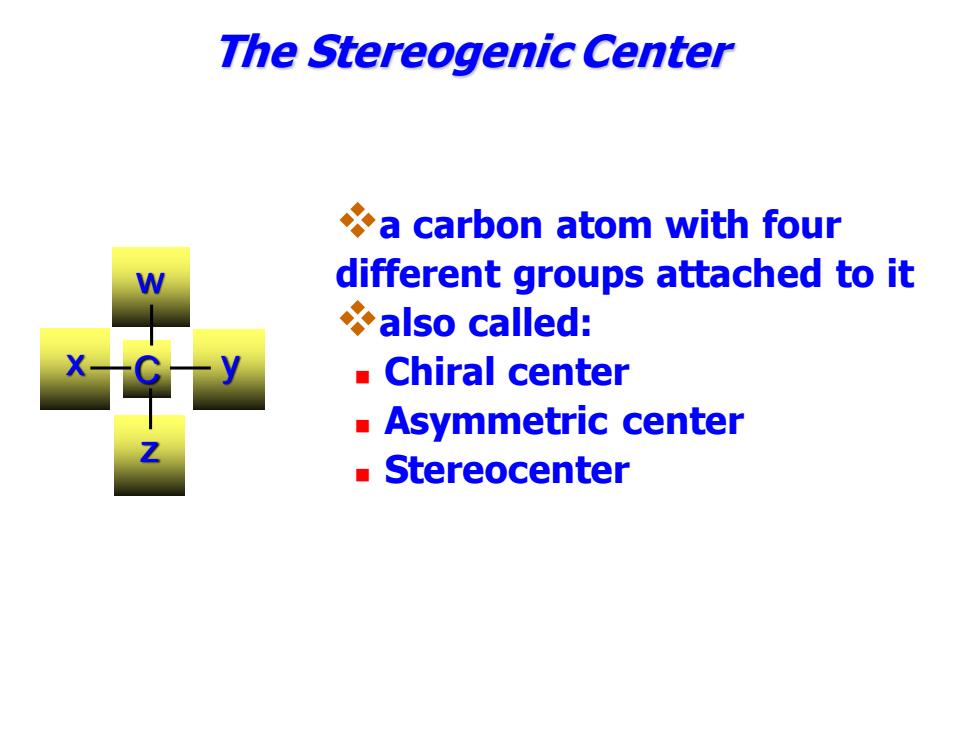
The Stereogenic Center a carbon atom with four different groups attached to it also called: Chiral center Asymmetric center Stereocenter
❖a carbon atom with four different groups attached to it ❖also called: ◼ Chiral center ◼ Asymmetric center ◼ Stereocenter The Stereogenic Center w x y z C

Chiral Carbons Tetrahedral carbons with 4 different attached groups are chiral. If there's only one chiral carbon in a molecule,its mirror image will be a different compound (enantiomer). mirror Copyright 2005 Pearson Prentice Hall.Inc
Chiral Carbons ❖Tetrahedral carbons with 4 different attached groups are chiral. ❖If there’s only one chiral carbon in a molecule, its mirror image will be a different compound (enantiomer)
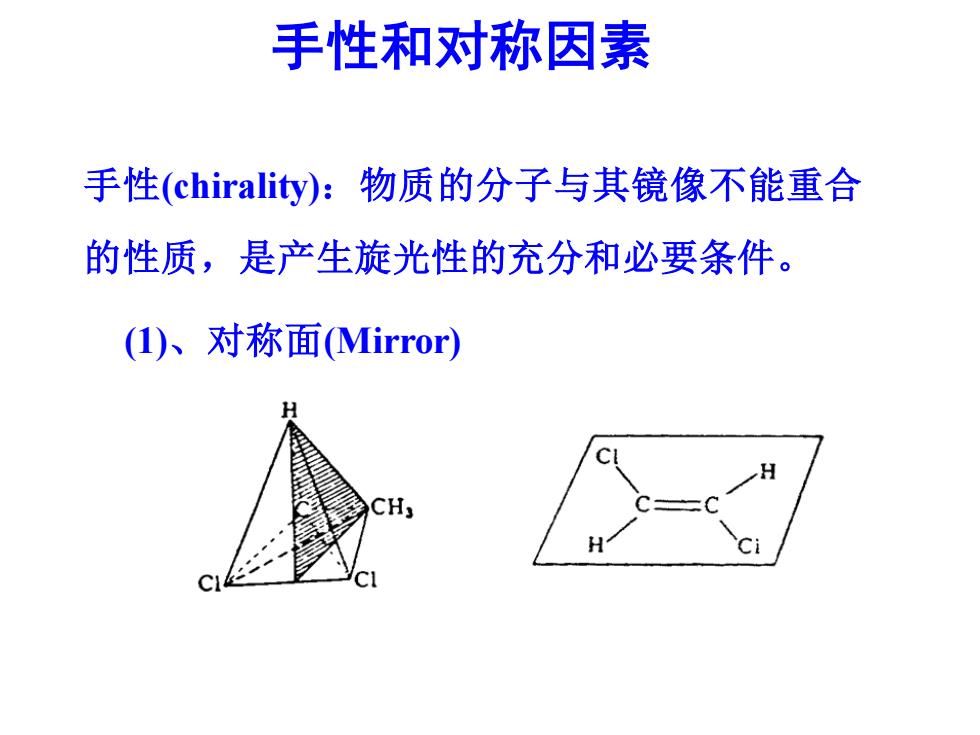
手性和对称因素 手性(chirality):物质的分子与其镜像不能重合 的性质,是产生旋光性的充分和必要条件。 (I)、对称面(Mirror)
手性和对称因素 手性(chirality):物质的分子与其镜像不能重合 的性质,是产生旋光性的充分和必要条件。 (1)、对称面(Mirror)