
Achiral Compounds rotate 180 rotate same as the first structure Take this mirror image and try to superimpose it on the one to the left matching all the atoms. Everything will match. When the images can be superposed,the compound is achiral 2013 Pearson Education,Inc. Chapter 5 11
© 2013 Pearson Education, Inc. Achiral Compounds Take this mirror image and try to superimpose it on the one to the left matching all the atoms. Everything will match. When the images can be superposed, the compound is achiral. Chapter 5 11
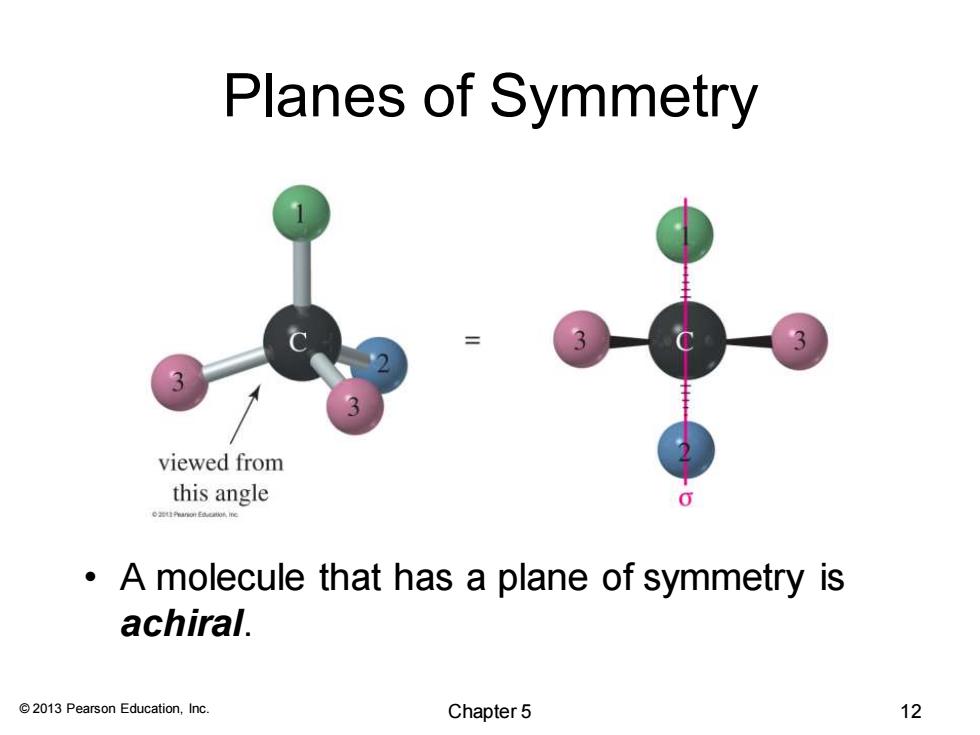
Planes of Symmetry 3 viewed from this angle 0 A molecule that has a plane of symmetry is achiral. 2013 Pearson Education,Inc. Chapter 5 12
© 2013 Pearson Education, Inc. Planes of Symmetry • A molecule that has a plane of symmetry is achiral. Chapter 5 12

Cis Cyclic Compounds 0 H H CI CI internal mirror plane of symmetry (o) Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry.Both structures above can be superimposed (they are identical to their mirror images). 2013 Pearson Education,Inc. Chapter 5 13
© 2013 Pearson Education, Inc. Cis Cyclic Compounds • Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry. Both structures above can be superimposed (they are identical to their mirror images). Chapter 5 13

Trans Cyclic Compounds not a plane enantiomers of symmetry H does not correspond different from the structure at left Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the images are nonsuperimposable and the molecule will have two enantiomers. 2013 Pearson Education,Inc. Chapter 5 14
© 2013 Pearson Education, Inc. Trans Cyclic Compounds • Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the images are nonsuperimposable and the molecule will have two enantiomers. Chapter 5 14

(R)and (S)Configuration OH HO 米 CH3 NH2 HN CH natural alanine unnatural alanine Both enantiomers of alanine receive the same name in the IUPAC system:2-aminopropanoic acid. Only one enantiomer is biologically active.In alanine only the enantiomer on the left can be metabolized by the enzyme. A way to distinguish between them is to use stereochemical modifiers(R)and (S). 2013 Pearson Education,Inc. Chapter 5 15
© 2013 Pearson Education, Inc. (R) and (S) Configuration • Both enantiomers of alanine receive the same name in the IUPAC system: 2-aminopropanoic acid. • Only one enantiomer is biologically active. In alanine only the enantiomer on the left can be metabolized by the enzyme. • A way to distinguish between them is to use stereochemical modifiers (R) and (S). Chapter 5 15