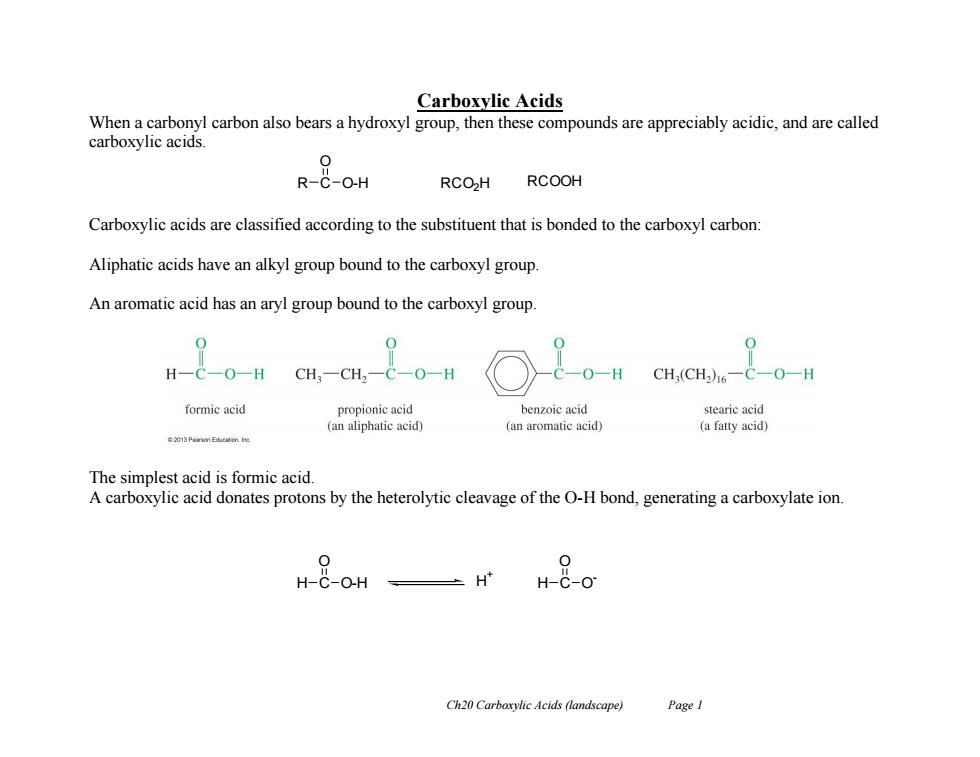
Carboxylic Acids When a carbonyl carbon also bears a hydroxyl group,then these compounds are appreciably acidic,and are called carboxylic acids. 0 R-C-O-H RCOH RCOOH Carboxylic acids are classified according to the substituent that is bonded to the carboxyl carbon: Aliphatic acids have an alkyl group bound to the carboxyl group. An aromatic acid has an aryl group bound to the carboxyl group 0 H-C-O-H CH:-CH,-C- -O-H C一O-H CH,(CH)6一C-O-H formic acid propionic acid benzoic acid stearic acid (an aliphatic acid) (an aromatic acid) (a fatty acid) ◆20aP3 Edicaton he The simplest acid is formic acid. A carboxylic acid donates protons by the heterolytic cleavage of the O-H bond,generating a carboxylate ion 0 H-C-O-H H H-C-O Ch20 Carboxylic Acids (landscape) PageI
Ch20 Carboxylic Acids (landscape) Page 1 Carboxylic Acids When a carbonyl carbon also bears a hydroxyl group, then these compounds are appreciably acidic, and are called carboxylic acids. Carboxylic acids are classified according to the substituent that is bonded to the carboxyl carbon: Aliphatic acids have an alkyl group bound to the carboxyl group. An aromatic acid has an aryl group bound to the carboxyl group. The simplest acid is formic acid. A carboxylic acid donates protons by the heterolytic cleavage of the O-H bond, generating a carboxylate ion. R C O O-H RCO2H RCOOH H C O O-H H C O O H + -
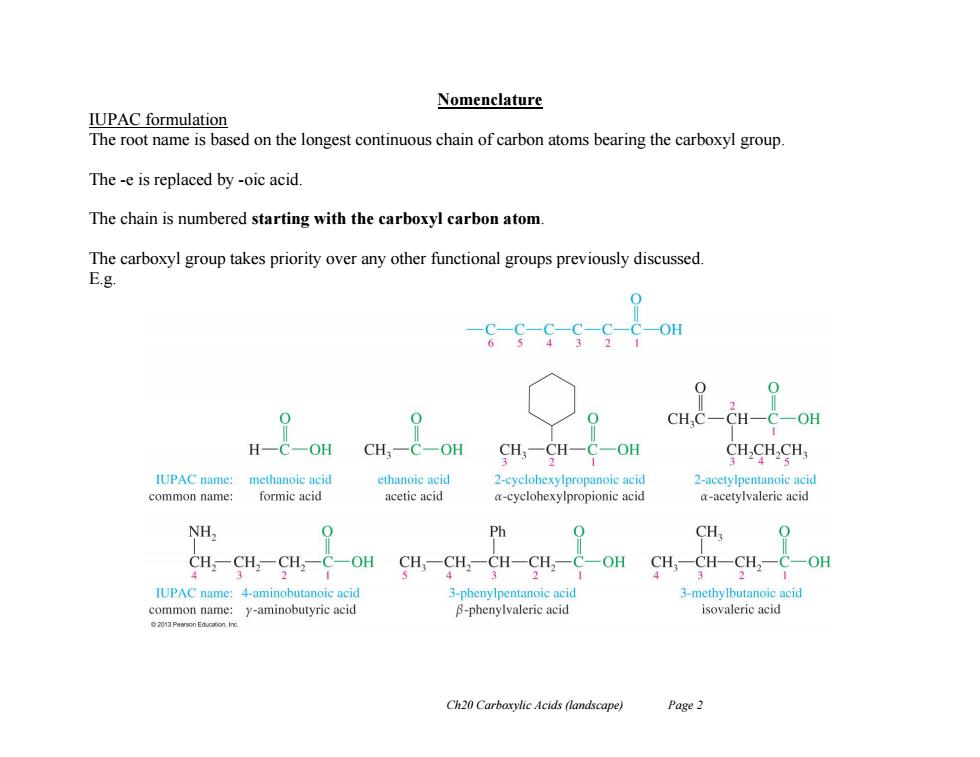
Nomenclature IUPAC formulation The root name is based on the longest continuous chain of carbon atoms bearing the carboxyl group. The -e is replaced by -oic acid The chain is numbered starting with the carboxyl carbon atom The carboxyl group takes priority over any other functional groups previously discussed. E.g. c-s-G6-s c CH,C-CH-C-OH H一C-OH CH,-C-OH CH3-CH-C-OH CH,CH,CH IUPAC name: methanoic acid ethanoic acid 2-cyclohexylpropanoic acid 2-acetylpentanoic acid common name: formic acid acetic acid a-cyclohexylpropionic acid a-acetylvaleric acid NH, Ph CH, -OH CH,-CH,一CH-CH2-C-OH CH-CH-H,一 -OH IUPAC name:4-aminobutanoic acid 3-phenylpentanoic acid 3-methylbutanoic acid common name:y-aminobutyric acid B-phenylvaleric acid isovaleric acid 2013 Peorson Educooon Inc Ch20 Carboxylic Acids (landscape) Page 2
Ch20 Carboxylic Acids (landscape) Page 2 Nomenclature IUPAC formulation The root name is based on the longest continuous chain of carbon atoms bearing the carboxyl group. The -e is replaced by -oic acid. The chain is numbered starting with the carboxyl carbon atom. The carboxyl group takes priority over any other functional groups previously discussed. E.g
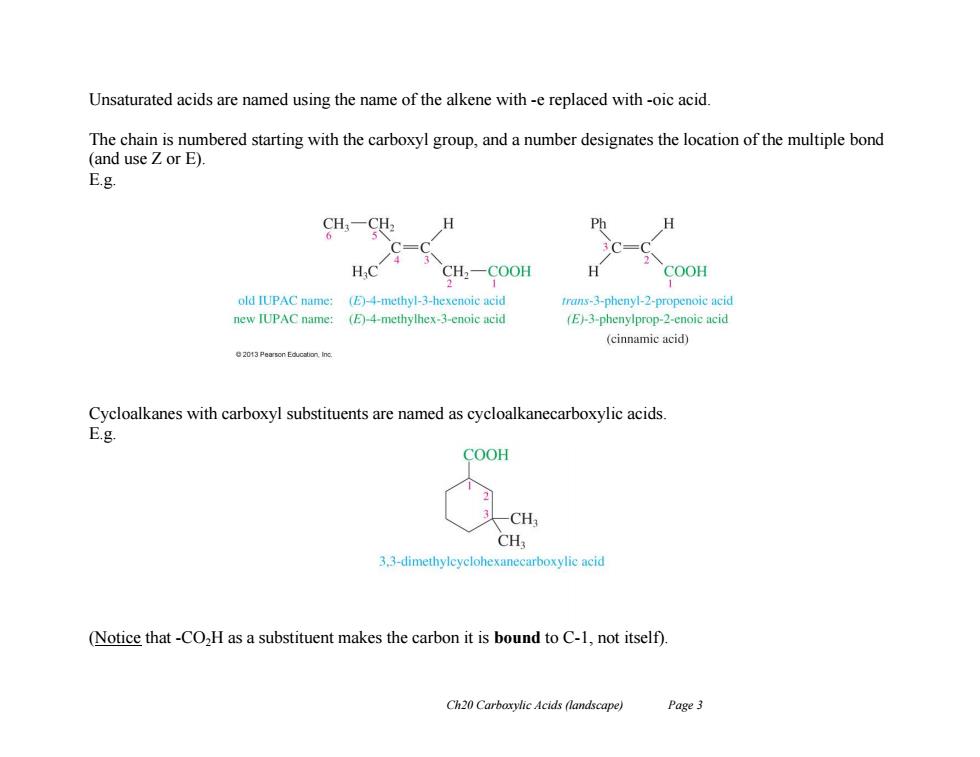
Unsaturated acids are named using the name of the alkene with -e replaced with -oic acid. The chain is numbered starting with the carboxyl group,and a number designates the location of the multiple bond (and use Z or E). E.g. P CH2-COOH COOH old IUPAC name:()-4-methyl-3-hexenoic acid trans-3-phenyl-2-propenoic acid new IUPAC name: (E)-4-methylhex-3-enoic acid (E)-3-phenylprop-2-enoic acid (cinnamic acid) 20t3 Pearson Educslion inc. Cycloalkanes with carboxyl substituents are named as cycloalkanecarboxylic acids. E.g COOH -CH CH 3.3-dimethyleyclohexanecarboxylic acid (Notice that -CO,H as a substituent makes the carbon it is bound to C-1,not itself). Ch20 Carboxylic Acids (landscape) Page 3
Ch20 Carboxylic Acids (landscape) Page 3 Unsaturated acids are named using the name of the alkene with -e replaced with -oic acid. The chain is numbered starting with the carboxyl group, and a number designates the location of the multiple bond (and use Z or E). E.g. Cycloalkanes with carboxyl substituents are named as cycloalkanecarboxylic acids. E.g. (Notice that -CO2H as a substituent makes the carbon it is bound to C-1, not itself)
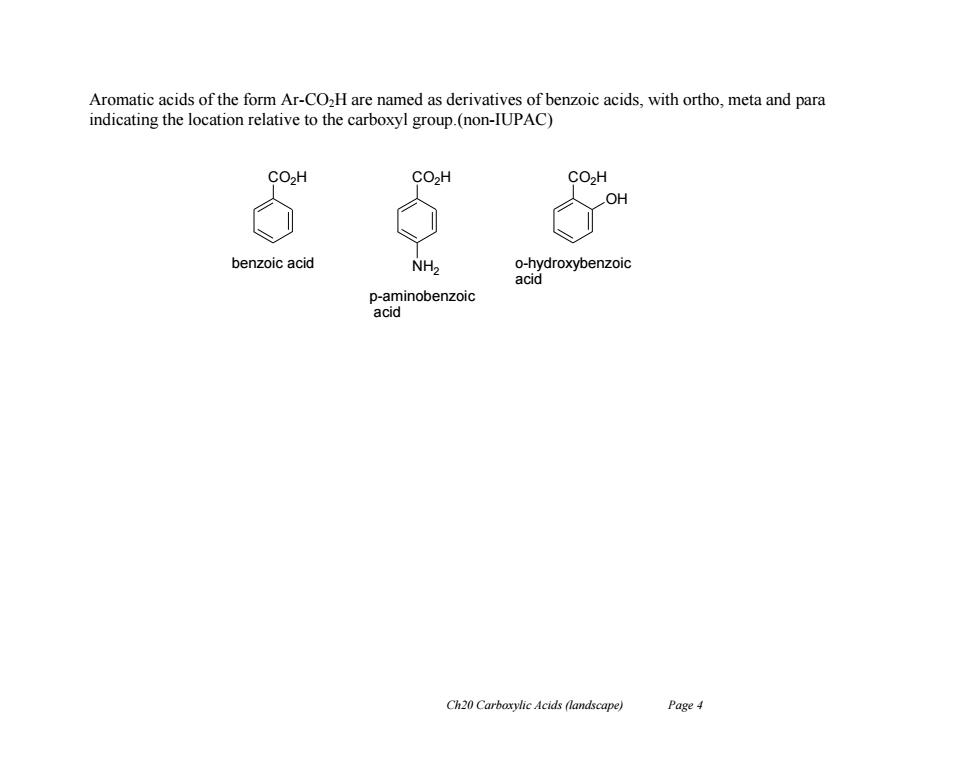
Aromatic acids of the form Ar-CO2H are named as derivatives of benzoic acids,with ortho,meta and para indicating the location relative to the carboxyl group.(non-IUPAC) CO2H CO2H CO>H OH benzoic acid NH> o-hydroxybenzoic acid p-aminobenzoic acid Ch20 Carboxylic Acids (landscape) Page 4
Ch20 Carboxylic Acids (landscape) Page 4 Aromatic acids of the form Ar-CO2H are named as derivatives of benzoic acids, with ortho, meta and para indicating the location relative to the carboxyl group.(non-IUPAC) CO2H CO2H CO2H benzoic acid p-aminobenzoic acid o-hydroxybenzoic acid NH2 OH
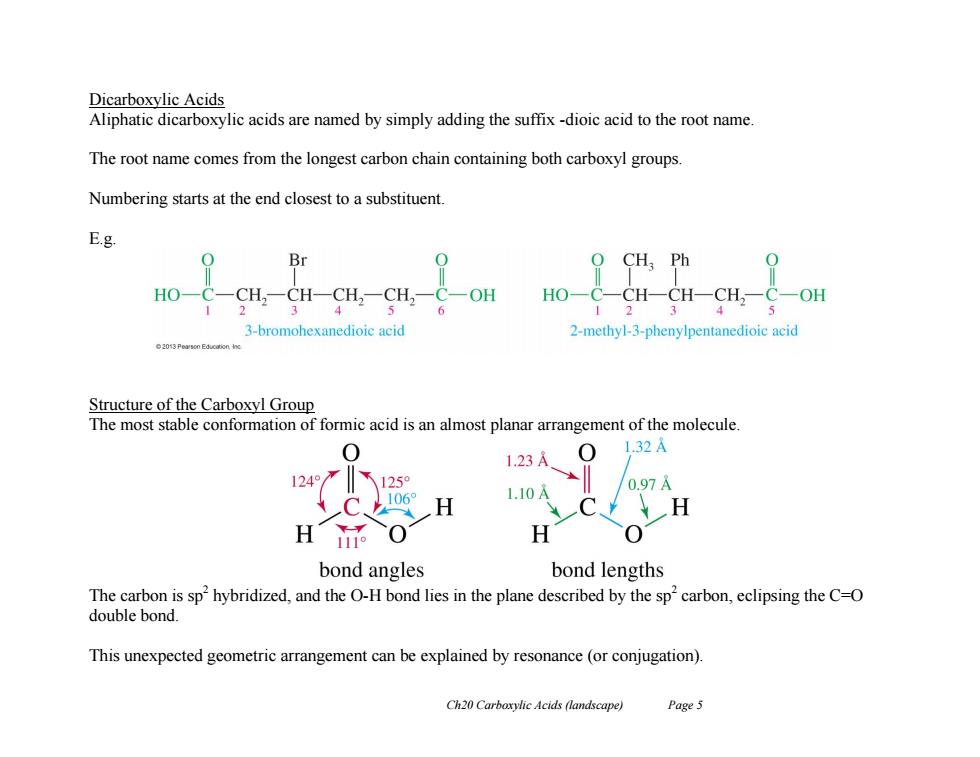
Dicarboxylic Acids Aliphatic dicarboxylic acids are named by simply adding the suffix -dioic acid to the root name. The root name comes from the longest carbon chain containing both carboxyl groups. Numbering starts at the end closest to a substituent. E.g. Br O CH Ph HO-C-CH2-CH-CH,-CH,-C-OH 4 HO-C一SH-CH-CH,-C-OH 1 3-bromohexanedioic acid 2-methyl-3-phenylpentanedioic acid Structure of the Carboxyl Group The most stable conformation of formic acid is an almost planar arrangement of the molecule. 0 1.32A 1.23A 1245125 1.10A 0.97A 1106° H H 0 bond angles bond lengths The carbon is sp2hybridized,and the O-H bond lies in the plane described by the sp'carbon,eclipsing the C=O double bond. This unexpected geometric arrangement can be explained by resonance(or conjugation). Ch20 Carboxylic Acids (landscape) Page 5
Ch20 Carboxylic Acids (landscape) Page 5 Dicarboxylic Acids Aliphatic dicarboxylic acids are named by simply adding the suffix -dioic acid to the root name. The root name comes from the longest carbon chain containing both carboxyl groups. Numbering starts at the end closest to a substituent. E.g. Structure of the Carboxyl Group The most stable conformation of formic acid is an almost planar arrangement of the molecule. The carbon is sp2 hybridized, and the O-H bond lies in the plane described by the sp2 carbon, eclipsing the C=O double bond. This unexpected geometric arrangement can be explained by resonance (or conjugation)