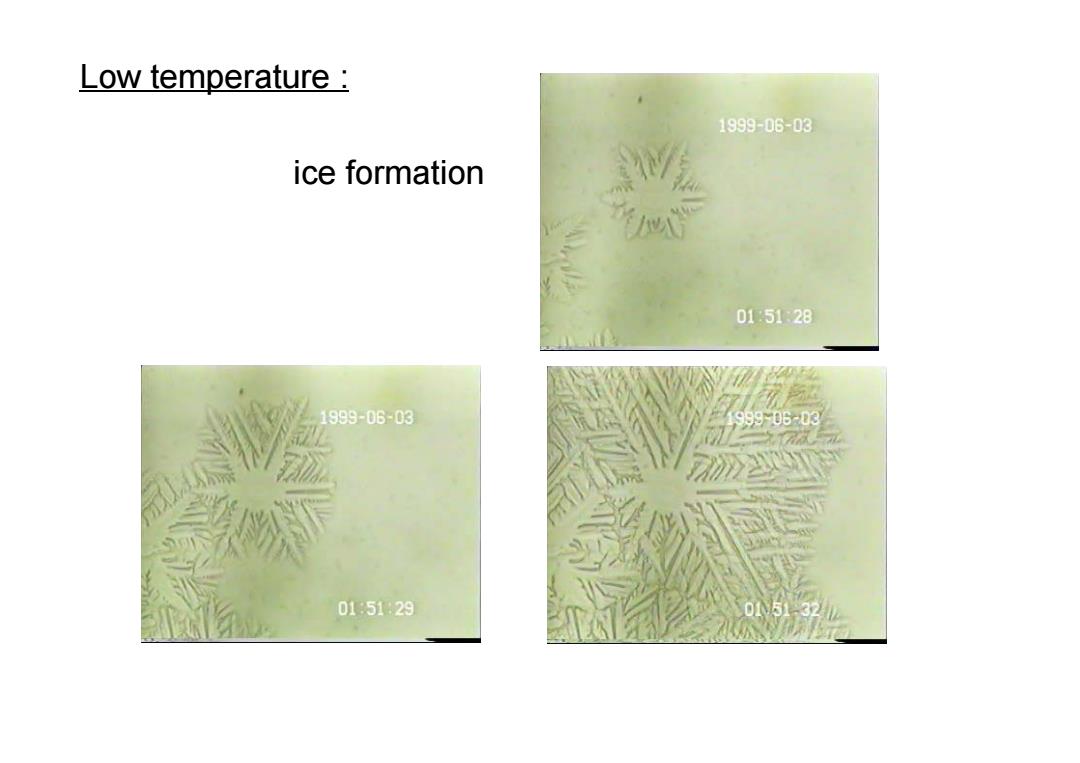
Low temperature: 1999-06-03 ice formation 0151:28 1999-06-03 99906-03 015129 0151-327
Low temperature : ice formation
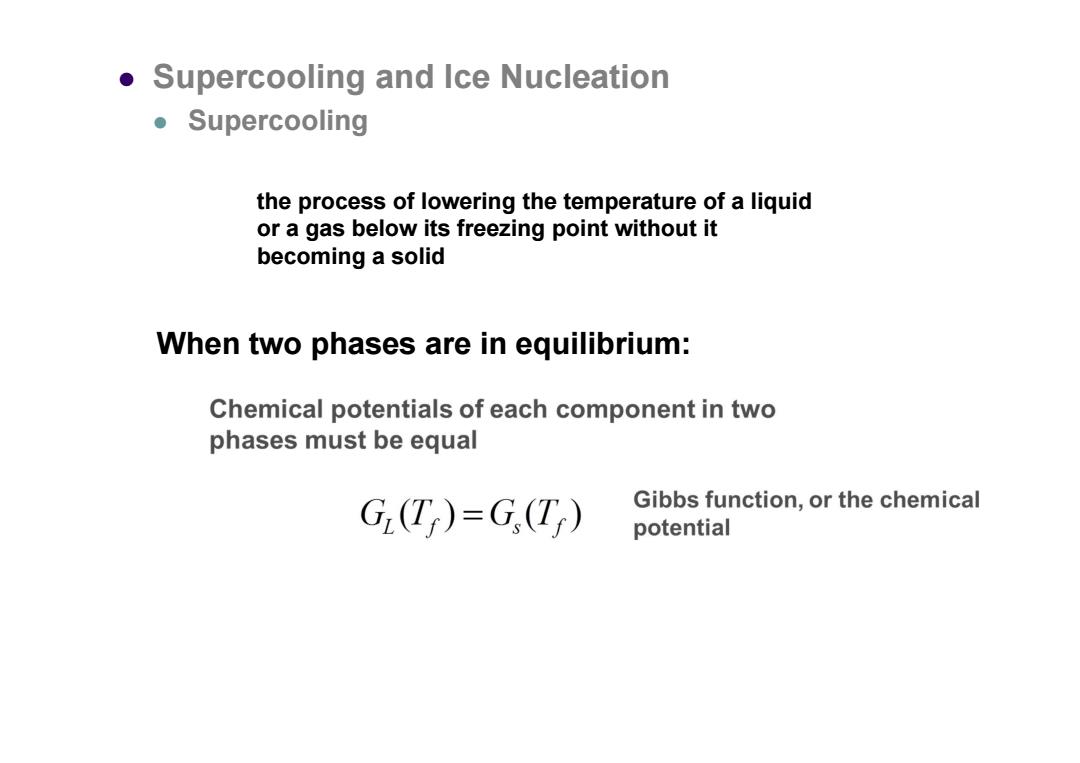
Supercooling and Ice Nucleation ●Supercooling the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid When two phases are in equilibrium: Chemical potentials of each component in two phases must be equal G(T)=G(T) Gibbs function,or the chemical potential
the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid Supercooling and Ice Nucleation Supercooling When two phases are in equilibrium:

Super cooling degree AT=TI-To At supercooling: G(T。)>G,(T。) △G(T。)=G(T。)-G,(T。) ≡△TAS(Tf) =Lr△T1Tf
T Tf To ( ) ( ) GL To Gs To f f f o L o s o L T T T S T G T G T G T / ( ) ( ) ( ) ( ) Super cooling degree At supercooling:

Ice Nucleation with different supercooling Cooling from 10C to -40C with Seed ice at-4C,hold at- cooling rate-20C/min,picture 40C/min for 2min,then cooling taken at-40C.(ice forms at to -400C at-20C/min.picture -10C) taken at-40C.(ice forms at -4C)
Cooling from 10oC to -40oC with cooling rate -20oC/min, picture taken at -40oC. (ice forms at -10oC) Seed ice at -4oC, hold at - 4oC/min for 2min, then cooling to -40oC at -20oC/min. picture taken at -40oC. (ice forms at -4oC) Ice Nucleation with different supercooling

When there are cells in the water solution Cross-Section of an Animal Cell Cell wall Cel Membrine Centrose开e mane Encoolasmc Ly多1B程号 Cytopiasm etculum 只t09me等 Rough ER Nuceus Nucleotus 5weo中ER Nucieclus Nuclear Me作由r法ne 风be4anet Cytcplasm Mtochoneron . PLANT CELL STRUCTURE