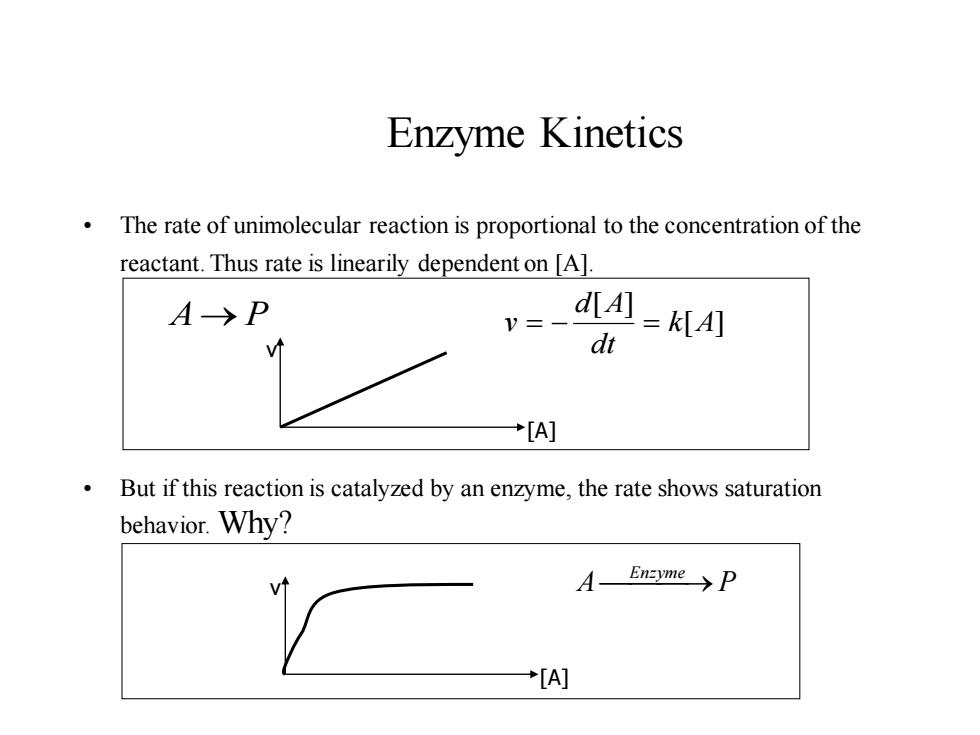
Enzyme Kinetics The rate of unimolecular reaction is proportional to the concentration of the reactant.Thus rate is linearily dependent on [A]. A→P dA]=KIA] dt +[A] But if this reaction is catalyzed by an enzyme,the rate shows saturation behavior.Why? A Enzyme→P +[A]
Enzyme Kinetics • The rate of unimolecular reaction is proportional to the concentration of the reactant. Thus rate is linearily dependent on [A]. • But if this reaction is catalyzed by an enzyme, the rate shows saturation behavior. Why? A→ P [ ] [ ] k A dt d A v = − = v [A] A P v ⎯Enzyme ⎯⎯→ [A]

The Mechaelis-Menten Equation You need to know how this is derived E+S点S冬E+P This is the complete chemical formula for an enzyme-catalyzed(E)reaction of substrate(S)and product(P); 。 Mechaelis-Menten equation describes the relationship between reaction rate and substrate concentration.It can explain the saturation behavior in catalyzed reactions as shown in the previous slide. Mechaelis-Menten equation is derived based on the following three conditions: 1)State steady assumption, 2)Initial velocity assumption; 3)Rate law
The Mechaelis-Menten Equation You need to know how this is derived • This is the complete chemical formula for an enzyme-catalyzed (E) reaction of substrate(S) and product(P); • Mechaelis-Menten equation describes the relationship between reaction rate and substrate concentration. It can explain the saturation behavior in catalyzed reactions as shown in the previous slide. • Mechaelis-Menten equation is derived based on the following three conditions: 1) State steady assumption; 2) Initial velocity assumption; 3) Rate law. E + S ES E + P k1 K-1 k2 K-2

Steady State Assumption E+S,包BS号E+P Steady state is defined as the state during which the enzyme-substrate complex,[ES],remains constant,or d[ES]=0 dt Pre-steady state is the state during which [ES]builds up,usually very fast; MM equation concerns the reaction rate that is measured only when the steady state has reached
Steady State Assumption • Steady state is defined as the state during which the enzyme-substrate complex, [ES], remains constant, or • Pre-steady state is the state during which [ES] builds up, usually very fast; • MM equation concerns the reaction rate that is measured only when the steady state has reached. 0 [ ] − = dt d ES E + S ES E + P k1 k2 K-2

Initial Velocity Assumption E+S名S冬E+P In the beginning of the reaction,there is very little product,or [P]is small.So the amount of [ES]contributed by E+P is negligible. Thus,the MM equation concerns the reaction rate that is measured during early reaction period. In which case,the enzyme catalyzed reaction can be modified to: E+S →ES E+P K
Initial Velocity Assumption • In the beginning of the reaction, there is very little product, or [P] is small. So the amount of [ES] contributed by E+P is negligible. • Thus, the MM equation concerns the reaction rate that is measured during early reaction period. • In which case, the enzyme catalyzed reaction can be modified to: • K-1 E + S ES E + P k1 K-1 k2 E + S ES E + P k1 k2 K-2