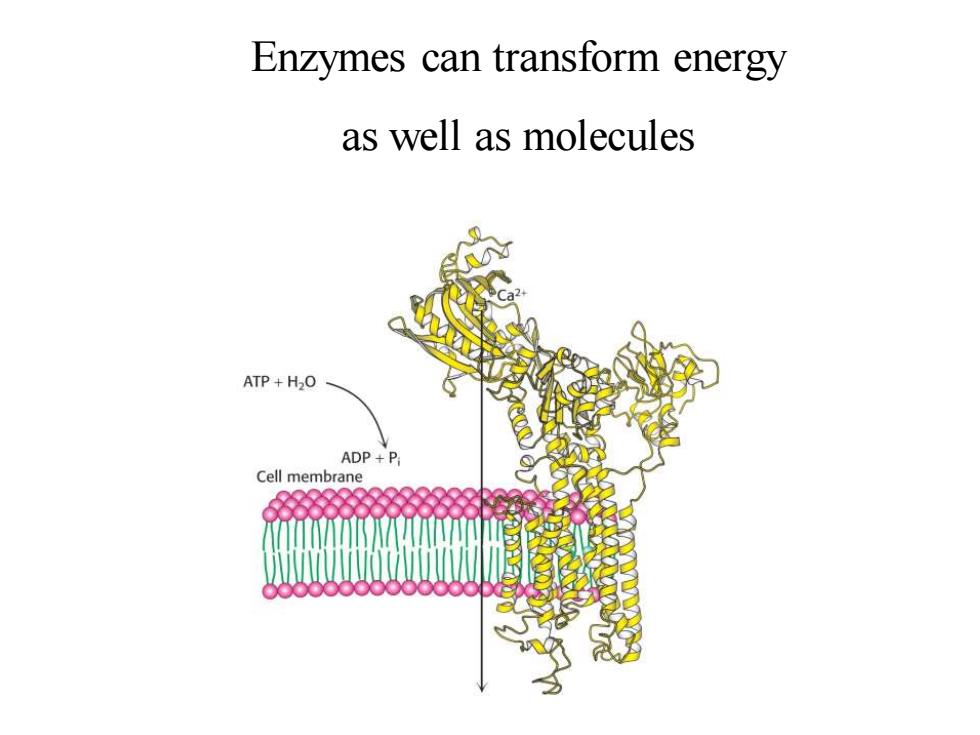
Enzymes can transform energy as well as molecules ATP+H2O ADP+P Cell membrane
Enzymes can transform energy as well as molecules

8.2 Introduction to Enzyme Kinetics Kinetics concerns with the rates of chemical reaction. Enzyme kinetics addresses the biological roles of enzymatic catalysts and quantify the remarkable function of biological enz☑ymes, Enzyme kinetics information can be exploited to control and manipulate the course of metabolic events.Pharmaceuticals or drugs are often special inhibitors targeted at a particular enzyme.Thus the science of pharmacology relies on such information
8.2 Introduction to Enzyme Kinetics • Kinetics concerns with the rates of chemical reaction. Enzyme kinetics addresses the biological roles of enzymatic catalysts and quantify the remarkable function of biological enzymes; • Enzyme kinetics information can be exploited to control and manipulate the course of metabolic events. Pharmaceuticals or drugs are often special inhibitors targeted at a particular enzyme. Thus the science of pharmacology relies on such information

8.3 Kinetics of Enzyme-Catalyzed Reaction The Rate Law of Unimolecular reactions(review) Consider a reaction ofoverall stoichiometry, A->P The rate,or velocity,v of this reaction is the amount of P formed or the amount of A consumed per unit time.Thus: V= d[P] d[A] Or V= dt dt Rate law states that: v=- (=kA] dt Where k is rate constant.v is a function of [A]to the first power,or the first order.k is called first order constant
8.3 Kinetics of Enzyme-Catalyzed Reaction The Rate Law of Unimolecular reactions (review) • Consider a reaction of overall stoichiometry, The rate, or velocity, v of this reaction is the amount of P formed or the amount of A consumed per unit time. Thus: Rate law states that: Where k is rate constant. v is a function of [A] to the first power, or the first order. k is called first order constant. dt d A or v dt d P v [ ] [ ] = = − A→ P [ ] [ ] k A dt d A v = − =
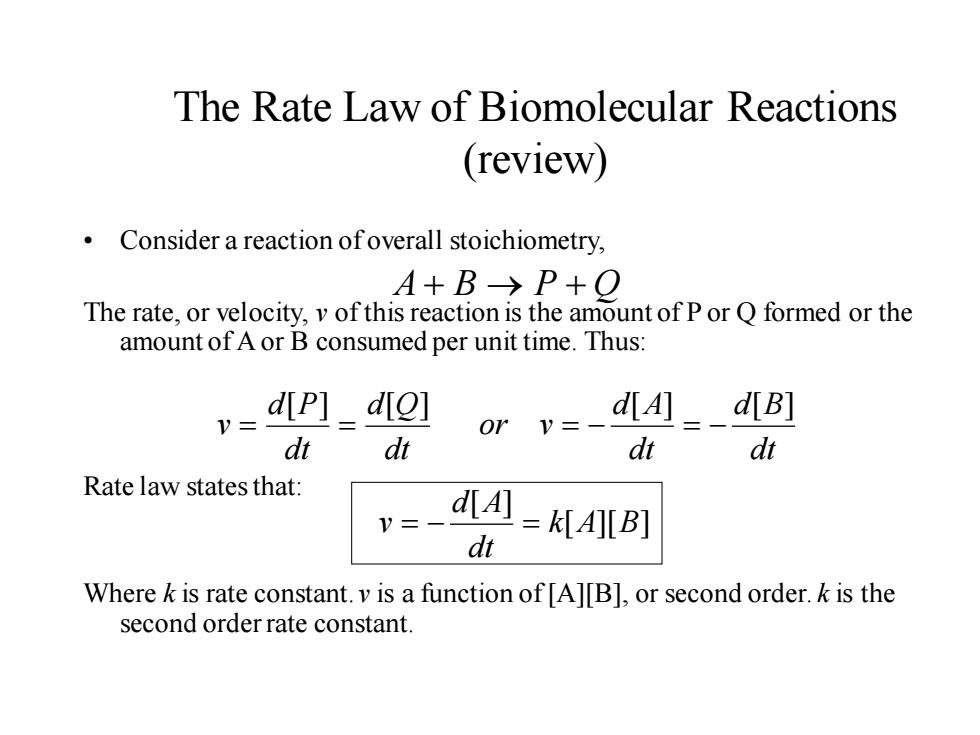
The Rate Law of Biomolecular Reactions (review) Consider a reaction ofoverall stoichiometry, A+B→P+O The rate,or velocity,v of this reaction is the amount of P or Q formed or the amount of A or B consumed per unit time.Thus: dip]_diol dt dt or v=- d[A]d[B] dt dt Rate law states that: d[A =KIAl[B] dt Where k is rate constant.v is a function of [A][B],or second order.k is the second order rate constant
The Rate Law of Biomolecular Reactions (review) • Consider a reaction of overall stoichiometry, The rate, or velocity, v of this reaction is the amount of P or Q formed or the amount of A or B consumed per unit time. Thus: Rate law states that: Where k is rate constant. v is a function of [A][B], or second order. k is the second order rate constant. dt d B dt d A or v dt d Q dt d P v [ ] [ ] [ ] [ ] = = = − = − A + B → P + Q [ ][ ] [ ] k A B dt d A v = − =
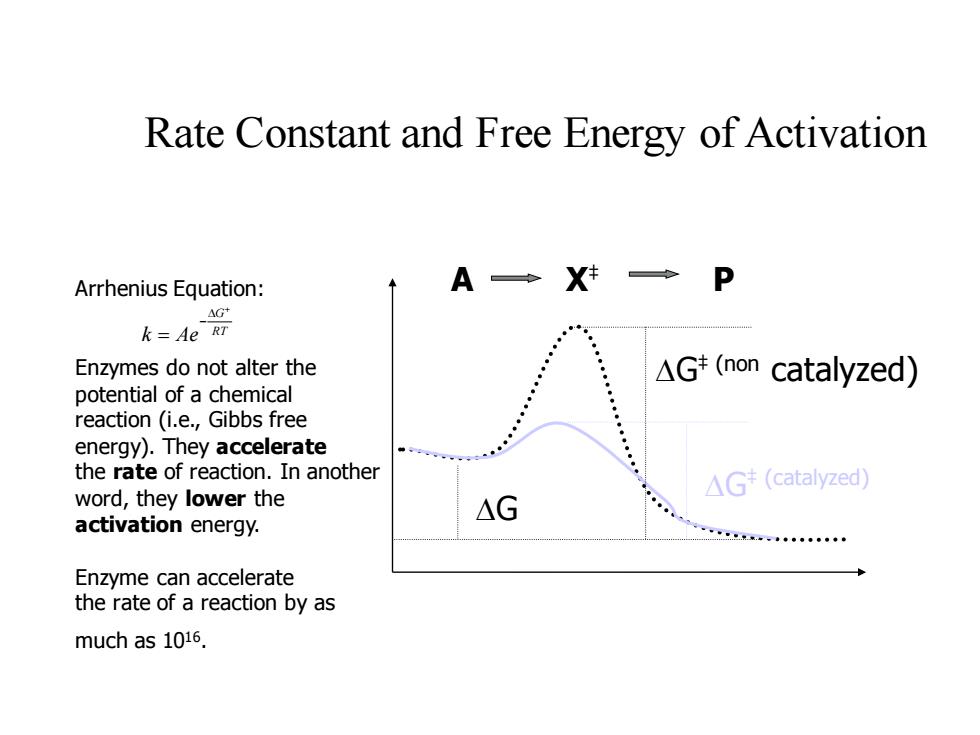
Rate Constant and Free Energy of Activation Arrhenius Equation: A◆X→P AG+ k=Ae RT Enzymes do not alter the △Gt(non catalyzed) potential of a chemical reaction (i.e.,Gibbs free energy).They accelerate the rate of reaction.In another △G(catalyzed word,they lower the △G activation energy. 二k。◆ Enzyme can accelerate the rate of a reaction by as much as 1016
Rate Constant and Free Energy of Activation DG‡ (non catalyzed) DG‡ (catalyzed) DG Arrhenius Equation: A X‡ P Enzymes do not alter the potential of a chemical reaction (i.e., Gibbs free energy). They accelerate the rate of reaction. In another word, they lower the activation energy. Enzyme can accelerate the rate of a reaction by as much as 1016 . RT G k Ae + D − =