
优势构象 交叉式构象:三对氢原子距离最远,分字 的能量最低,是最稳定的构象,也称优势 构象。 重叠式构象的能量比交叉式大12.5 kJ/mo1,此能量差称为转动能垒 (torsional energy)。 乙烷构象转化中的能量变化 丁烷转化中的能量变化
优势构象 交叉式构象:三对氢原子距离最远,分子 的能量最低,是最稳定的构象,也称优势 构象 。 重叠式构象的能量比交叉式大 12. 5 kJ/mol ,此能量差称为 转动能垒 (torsional energy) 。 乙烷构象转化中的能量变化 丁烷转化中的能量变化

东林开 B3 Nomenclature,Structure Properties B3-1:Nomenclature 1.IUPAC命名法 (International Union of Pure and Applied Chemistry,1892,The Geneva Rules) Name has three parts Prefix which specifies the location of functional groups and other substituents in the molecule Parent selects the main part of the molecule and tells the number of carbon atoms Suffix identifies functional I group family it belongs to Prefix-Parent-Suffix Where are the How many What functional-group substituents? carbons? family?
B3 Nomenclature, Structure & B3 Nomenclature, Structure & Properties Properties B3-1: Nomenclature Nomenclature 1. IUPAC命名法(International Union of Pure and Applied Chemistry,1892,The Geneva Rules ) Name has three parts Prefix which specifies the location of functional groups and other substituents in the molecule Parent selects the main part of the molecule and tells the number of carbon atoms Suffix identifies functional group family it belongs to

林 后行 CH 0一C% Methane Ehane Propane Butane Pentane Heptane Octane Decane Fg 1.Nomenclature of simple alkanes
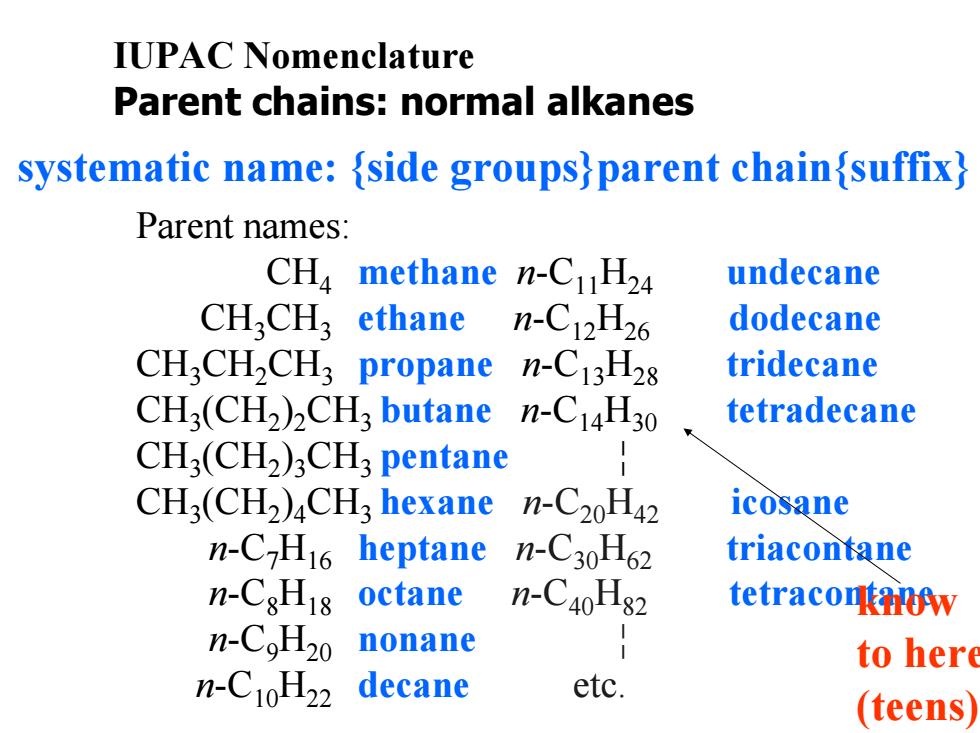
IUPAC Nomenclature Parent chains:normal alkanes systematic name:{side groups)parent chain{suffix) Parent names: CHa methane n-CH24 undecane CH,CH3 ethane n-C12H26 dodecane CH,CH2CH3 propane n-C13H28 tridecane CH3(CH2)CH3 butane n-C14H30 tetradecane CH3(CH2)3CH;pentane CH3(CH2)CH3 hexane n-C20H42 icosane n-C-H16 heptane n-C30H62 triacontane n-C8H18 octane n-C40H82 tetraconw n-CoH20 nonane to here n-C10H22 decane etc. (teens)
IUPAC Nomenclature Parent chains: normal alkanes systematic name: {side groups}parent chain{suffix} Parent names: CH 4 methane n-C11 H24 undecane CH 3CH 3 ethane n-C12 H26 dodecane CH 3CH 2CH 3 propane n-C13 H28 tridecane CH 3(CH 2 ) 2CH3 butane n-C14 H30 tetradecane CH 3(CH 2 ) 3CH3 pentane ¦ CH 3(CH 2 ) 4CH3 hexane n-C20 H42 icosane n-C 7 H16 heptane n-C30 H62 triacontane n-C 8 H18 octane n-C40 H82 tetracontane n-C 9 H20 nonane ¦ n-C10 H22 decane etc. know to her e (teens)

Extent of structural isomerism in alkanes Alkane No.of structural isomers Methane 1 Ethane 1 Propane 1 Butane 2 All known Pentane 3 Hexane 5 Decane 75 Pentadecane 4347 Eicosane 366,319 Triacontane 44×109 (C3oH62)
Extent of structural isomerism in alkanes Alkane No. of structural isomers Methane 1 Ethane 1 Propane 1 Butane 2 All known Pentane 3 Hexane 5 Decane 75 Pentadecane 4347 Eicosane 366,319 Triacontane 44 x 10 9 (C30 H62 )