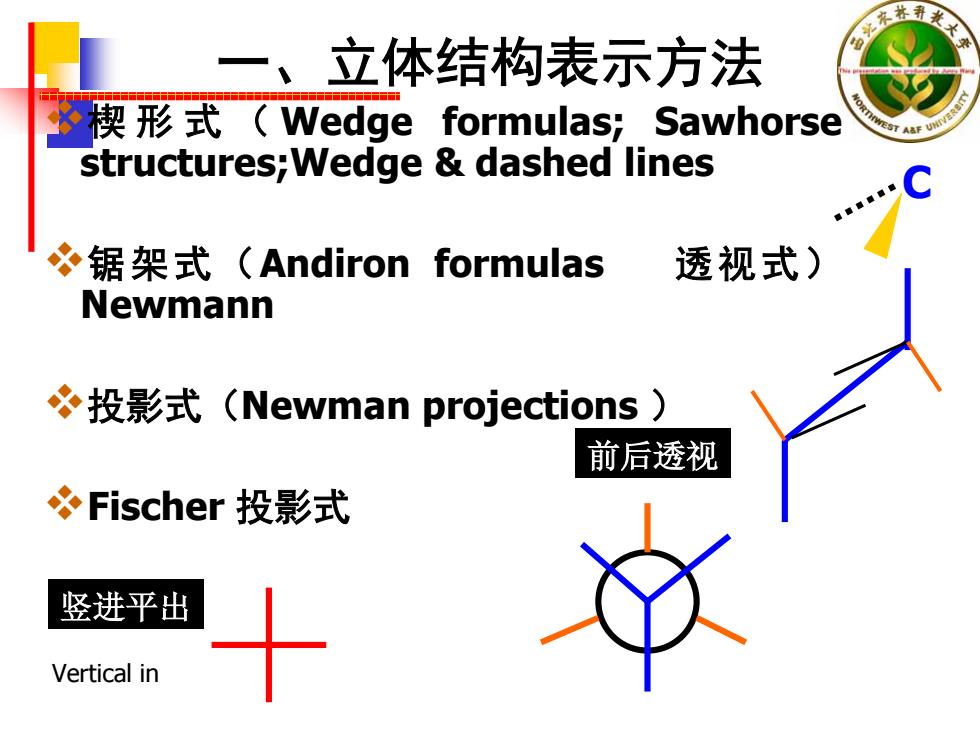
一、立体结构表示方法 楔形式(Wedge formulas;Sawhorse structures;Wedge dashed lines 锯架式(Andiron formulas 透视式) Newmann 投影式(Newman projections) 前后透视 Fischer投影式 竖进平出 Vertical in
一、立体结构表示方法 楔形式( Wedge formulas; Sawhorse structures;Wedge & dashed lines 锯架式(Andiron formulas 透视式) Newmann 投影式(Newman projections ) Fischer 投影式 C 竖进平出 前后透视 Vertical in
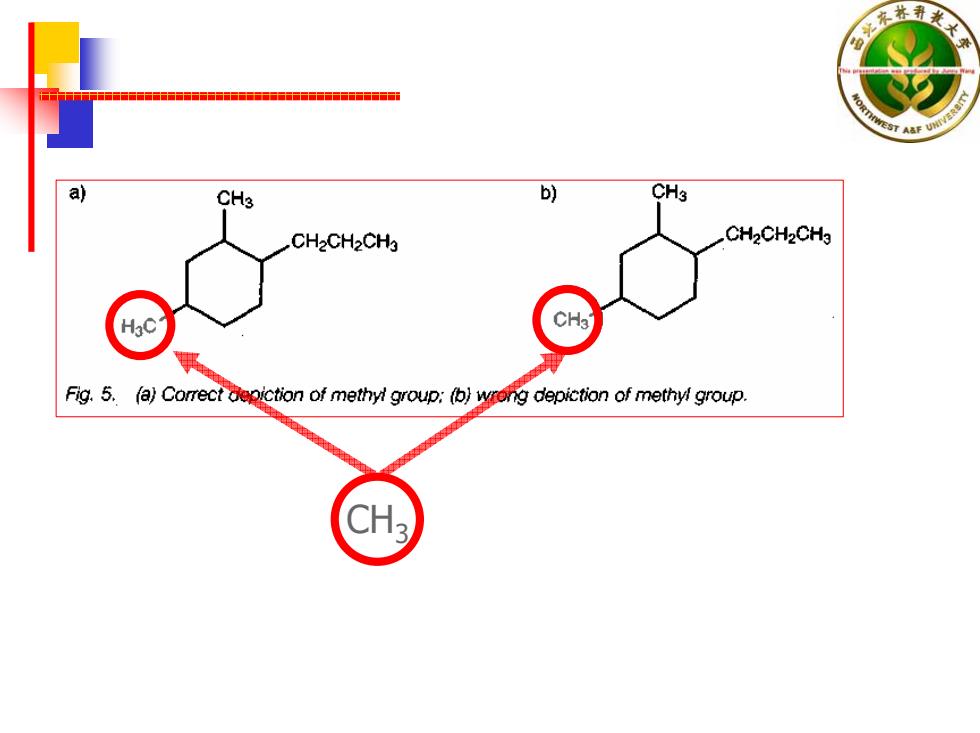
a) CH3 b) CHs CH2CH2CHg CH2CH2CHg HgC Fig.5.(a)Correct depiction of methyl group:(b)wrong depiction of methyl group
CH 3
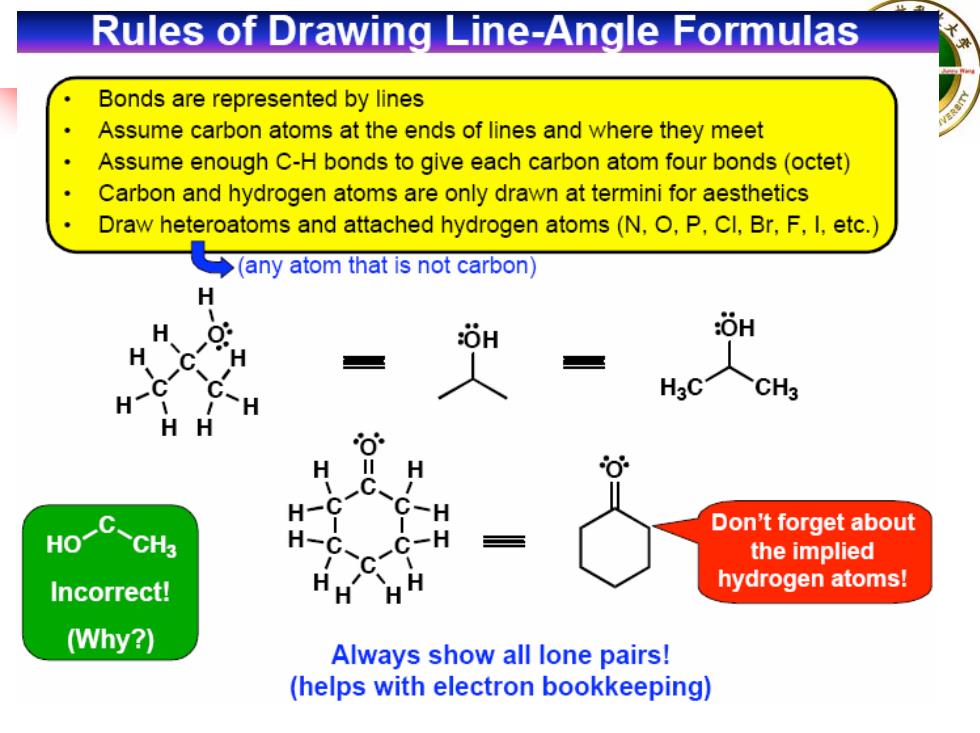
Rules of Drawing Line-Angle Formulas Bonds are represented by lines Assume carbon atoms at the ends of lines and where they meet Assume enough C-H bonds to give each carbon atom four bonds(octet) Carbon and hydrogen atoms are only drawn at termini for aesthetics Draw heteroatoms and attached hydrogen atoms(N,O,P,CI,Br,F,I,etc.) ◆(any atom that is not carbon)】 H H OH OH H G C C H H HgC CH3 HH H H C. H-C C-H HO-C CH3 Don't forget about H- the implied Incorrect! H hydrogen atoms! (Why?) Always show all lone pairs! (helps with electron bookkeeping)
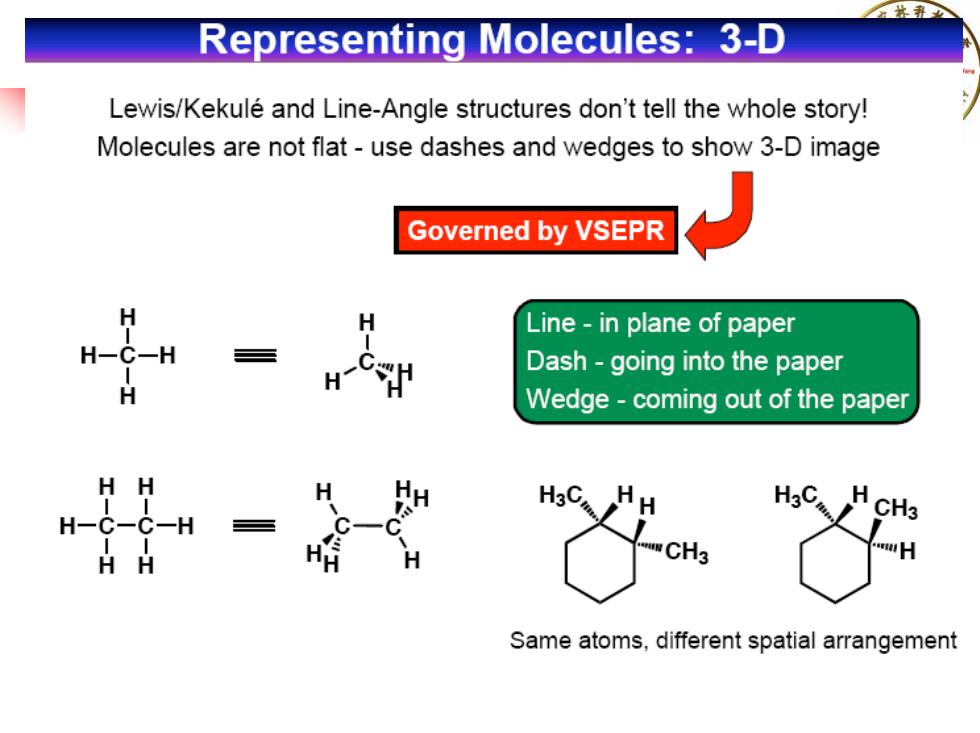
Representing Molecules:3-D Lewis/Kekule and Line-Angle structures don't tell the whole story! Molecules are not flat-use dashes and wedges to show 3-D image Governed by VSEPR Line-in plane of paper Dash-going into the paper H Wedge-coming out of the paper H H H3C. WCH3 Same atoms,different spatial arrangement

二、 构象与乙烷构象 十十 1.构象 Different arrangements of atoms that can be converted into one another by rotation about single bonds are called Conformations. 冬分子中的原子由于相互作用而在空间的排 布结构次序及立体形象称为构象 (Confromation) 构象(conformation):由于围绕单键 旋转而产生的分子中原子和原子团在空间 不同排列的形象;
二、 构象与乙烷构象 1. 构象 Different arrangements of atoms that can be converted into one another by rotation about single bonds are called Conformations. Conformations. 分子中的原子由于相互作用而在空间的排 布结构次序及立体形象称为构象 (Confromation) 构象(conformation):由于围绕单键 旋转而产生的分子中原子和原子团在空间 不同排列的形象;