上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 33 Chapter 7 Entropy (Section 7.1) Spring,4/26/2018 强 Prof.,Dr.Yonghua HUANG MMAMMA http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日G
Engineering Thermodynamics I Lecture 33 Spring, 4/26/2018 Prof., Dr. Yonghua HUANG Chapter 7 Entropy (Section 7.1) http://cc.sjtu.edu.cn/G2S/site/thermo.html
Directionality of processes Who determines the direction? Energy balance (1st law)? Entropy balance (2nd law)? HOT ·Together?? COFFEE Heat Spontaneous? 圈上游通大学 April 26,2018 2 SHANGHAI JLAO TONG UNIVERSITY
April 26, 2018 2 Directionality of processes Spontaneous? • Energy balance (1st law) ? • Entropy balance (2nd law)? • Together? Who determines the direction?
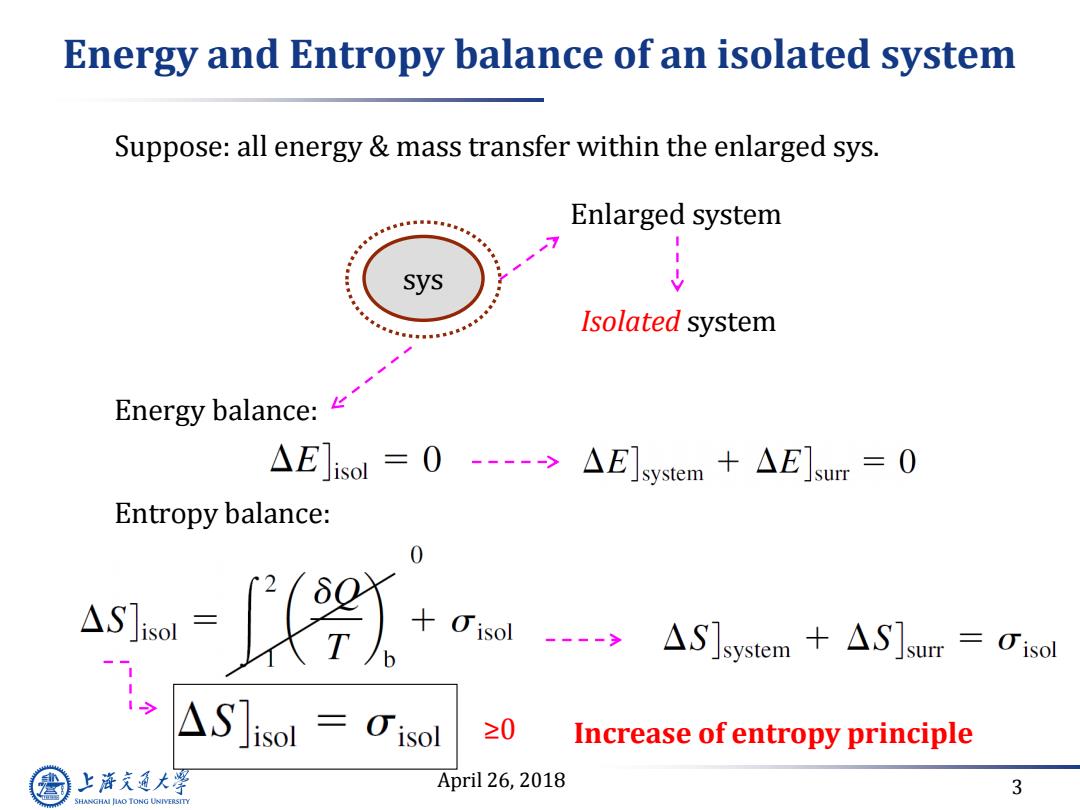
Energy and Entropy balance of an isolated system Suppose:all energy mass transfer within the enlarged sys. Enlarged system -7 sys Isolated system Energy balance: AE]isol=0-->△E]system+△E]sur=0 Entropy balance: agw= △S]isol=0isol ≥0 Increase of entropy principle 上游究通大浮 April 26,2018 3 SHANGHAI JIAO TONG UNIVERSITY
April 26, 2018 3 Energy and Entropy balance of an isolated system sys Enlarged system Isolated system Suppose: all energy & mass transfer within the enlarged sys. Energy balance: Entropy balance: ≥0 Increase of entropy principle
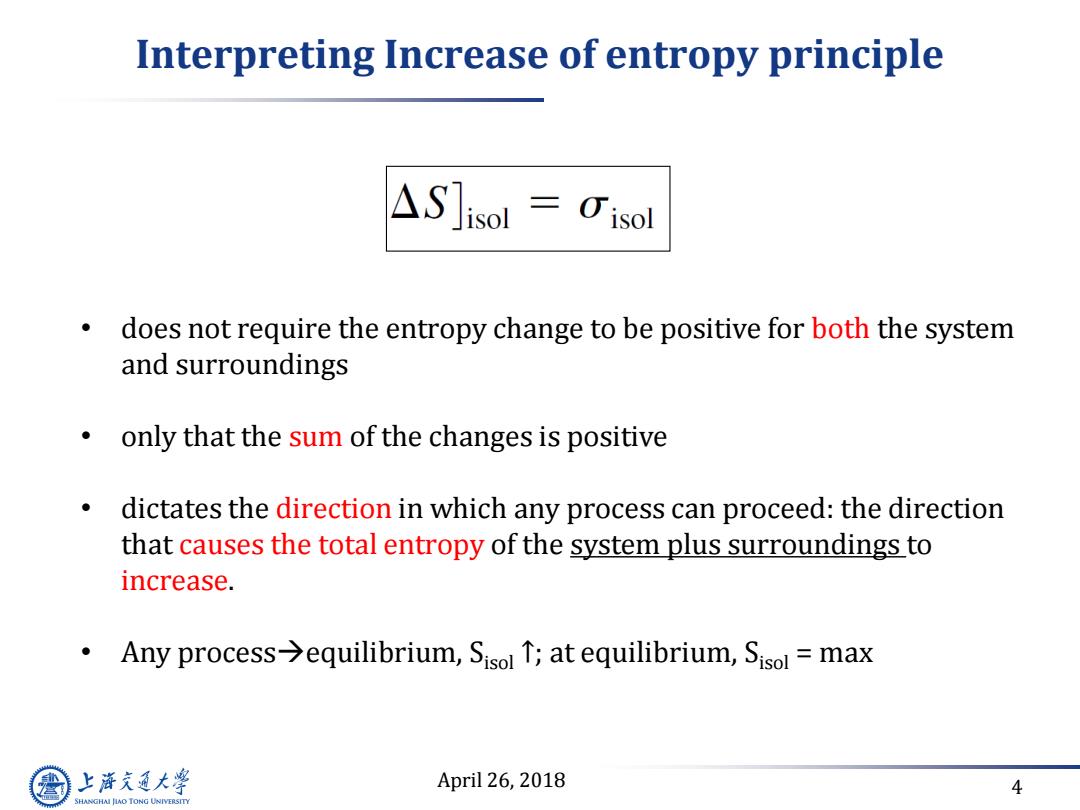
Interpreting Increase of entropy principle △S]isol=0isl does not require the entropy change to be positive for both the system and surroundings only that the sum of the changes is positive dictates the direction in which any process can proceed:the direction that causes the total entropy of the system plus surroundings to increase. Any process>equilibrium,Sisol T;at equilibrium,Sisol =max 上游通大学 April 26,2018 4 SHANGHAI JIAO TONG UNIVERSITY
April 26, 2018 4 Interpreting Increase of entropy principle • does not require the entropy change to be positive for both the system and surroundings • only that the sum of the changes is positive • dictates the direction in which any process can proceed: the direction that causes the total entropy of the system plus surroundings to increase. • Any processequilibrium, Sisol ↑; at equilibrium, Sisol = max
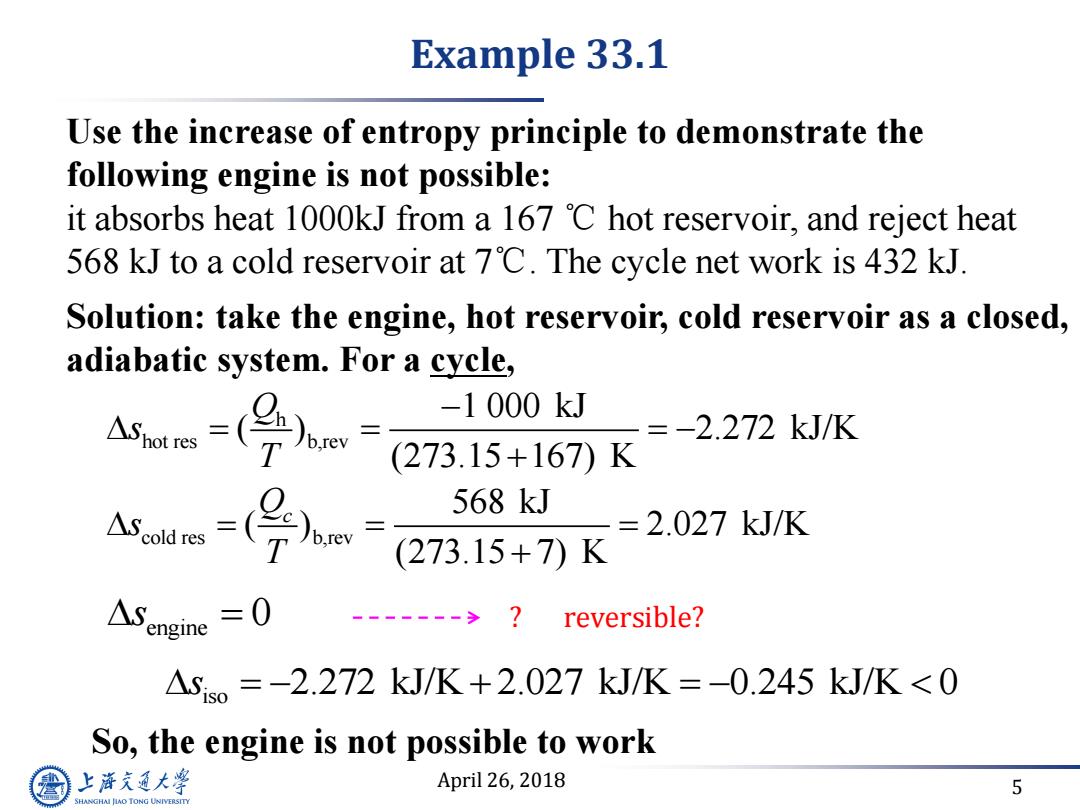
Example 33.1 Use the increase of entropy principle to demonstrate the following engine is not possible: it absorbs heat 1000kJ from a 167 C hot reservoir,and reject heat 568 kJ to a cold reservoir at 7'C.The cycle net work is 432 kJ. Solution:take the engine,hot reservoir,cold reservoir as a closed, adiabatic system.For a cycle, -1000kJ =-2.272kJ/K (273.15+167)K 568kJ =2.027kJ/K (273.15+7)K ASengine =0 reversible? △sso=-2.272kJ/K+2.027kJ/K=-0.245kJ/K<0 So,the engine is not possible to work 上游究通大学 April 26,2018 5 SHANGHAI JLAO TONG UNIVERSITY
April 26, 2018 5 Use the increase of entropy principle to demonstrate the following engine is not possible: it absorbs heat 1000kJ from a 167 ℃ hot reservoir, and reject heat 568 kJ to a cold reservoir at 7℃. The cycle net work is 432 kJ. hot res h b,rev 1 000 kJ ( ) 2.272 kJ/K (273.15 167) K Q s T So, the engine is not possible to work cold res b,rev 568 kJ ( ) 2.027 kJ/K (273.15 7) K Qc s T engine s 0 iso s 2.272 kJ/K 2.027 kJ/K 0.245 kJ/K 0 Solution: take the engine, hot reservoir, cold reservoir as a closed, adiabatic system. For a cycle, Example 33.1 ? reversible?