
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 48 Class Review and Final Exam. Spring,5/15/2018 Prof.,Dr.Yonghua HUANG 强 M http://cc.sjtu.edu.cn/G2S/site/thermo.html SHANG 1日 ERSITY
Engineering Thermodynamics I Lecture 48 Spring, 5/15/2018 Prof., Dr. Yonghua HUANG Class Review and Final Exam. http://cc.sjtu.edu.cn/G2S/site/thermo.html

Chap.1 System,boundary,surrounding. Closed system/open system/adiabatic system/ isolated system 国 What is property?Distinguish property from non- property.Characteristics of property. 国 Extensive and intensive properties Units for pressure,temperature,specific volume 上游充通大粤 May15,2018 2 HANGHAI HAO TONG LINIVERSITY
May 15, 2018 2 Chap. 1 System, boundary, surrounding. Closed system/open system/adiabatic system/ isolated system What is property? Distinguish property from non‐ property. Characteristics of property. Extensive and intensive properties Units for pressure, temperature, specific volume
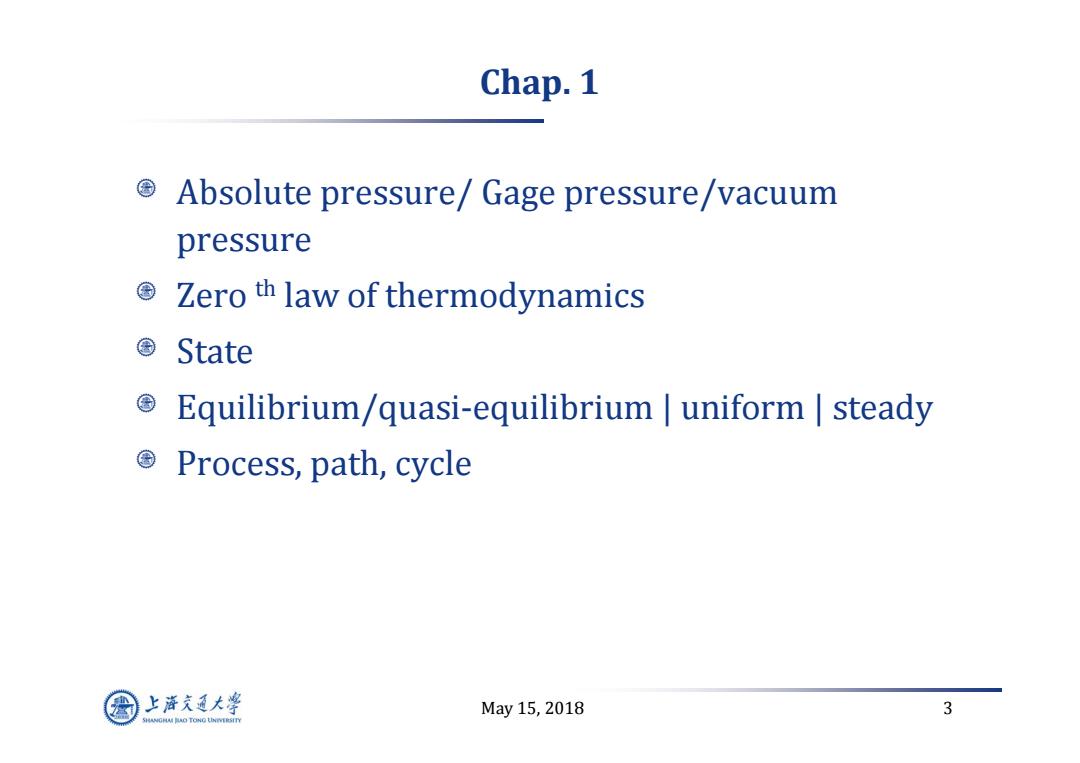
Chap.1 Absolute pressure/Gage pressure/vacuum pressure 国 Zero th law of thermodynamics State Equilibrium/quasi-equilibrium uniform steady 国 Process,path,cycle 上游充通大粤 May15,2018 3 SHANGHAI BAO TONG LINIERSITY
May 15, 2018 3 Chap. 1 Absolute pressure/ Gage pressure/vacuum pressure Zero th law of thermodynamics State Equilibrium/quasi‐equilibrium | uniform | steady Process, path, cycle

Chap.2 Energy potential energy (PE)kinetic energy (KE) 国 Change of energy pv Work/boundary work/shaft work/flow work... External force,non-quasi-equilibrium problem Sign (+/-definition of heat transfer and work 国 Internal energy... 上游充通大粤 May15,2018 4 HANGHAI HAO TONG LINIVERSITY
May 15, 2018 4 Chap. 2 Energy | potential energy (PE) |kinetic energy (KE) Change of energy Work/boundary work/ shaft work/ flow work… External force, non‐quasi‐equilibrium problem Sign (+/ ‐ ) definition of heat transfer and work Internal energy… pv
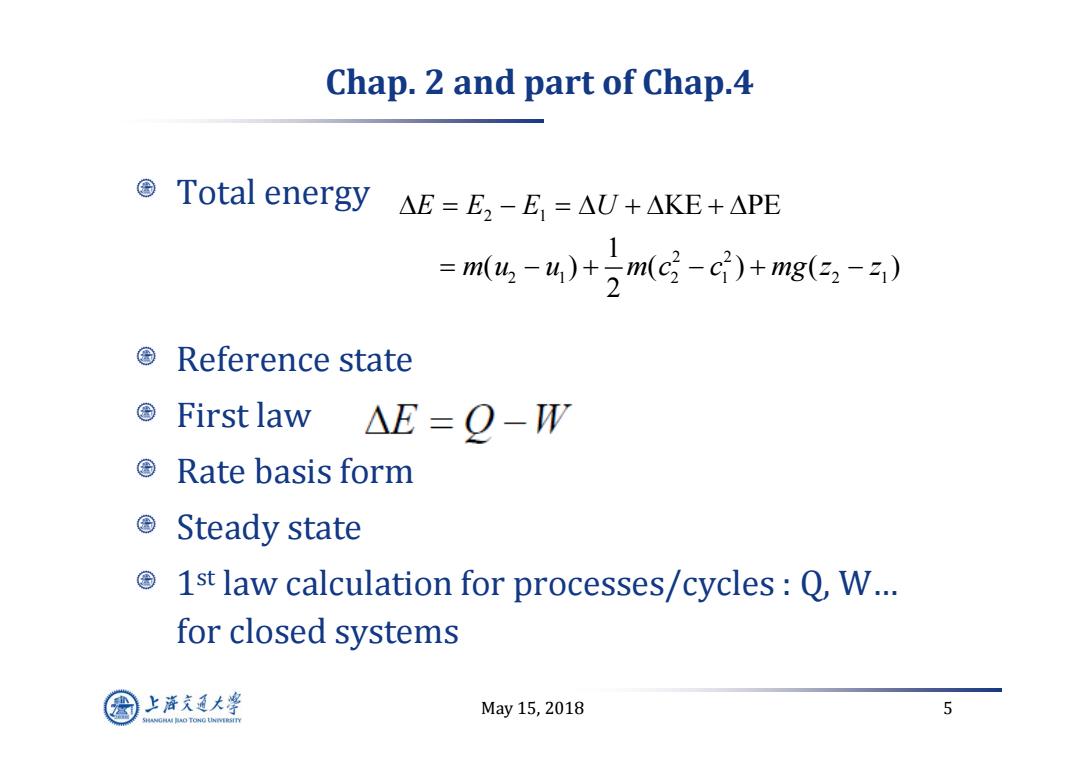
Chap.2 and part of Chap.4 ©Total energy△E=E,-E,=AU+AKE+APE =m,-4)+)m(c-c)+mg(32-2) Reference state First law △E=Q-W ©Rate basis form Steady state 国 1st law calculation for processes/cycles:Q,W... for closed systems 上游充通大¥ May15,2018 5 SHANGHAI BAO TONG LINIERSITY
May 15, 2018 5 Chap. 2 and part of Chap.4 Total energy Reference state First law Rate basis form Steady state 1st law calculation for processes/cycles : Q, W… for closed systems 2 1 2 2 21 21 21 KE PE 1 ( ) ( )( ) 2 EE E U mu u mc c m g z z