
Chap.3 Polytropic process for closed system py"constant P,v,T relations.For all gas?For ideal gas? Four particular cases:isobaric,isothermal, isentropic,isochoric ©△u,△h,w,q for polytropic process(n=1?nt1?) 圈△T,△u,△h,q,w trend on p-v diagram Applicability of those equations 上游充通大粤 May15,2018 11 HANGHAI HAO TONG LINIVERSITY
May 15, 2018 11 Chap. 3 Polytropic process for closed system P, v, T relations. For all gas? For ideal gas? Four particular cases: isobaric, isothermal, isentropic, isochoric Δu, Δh, w, q for polytropic process (n=1? n≠1?) ∆T, ∆u, ∆h, q, w trend on p‐v diagram n pV constant Applicability of those equations
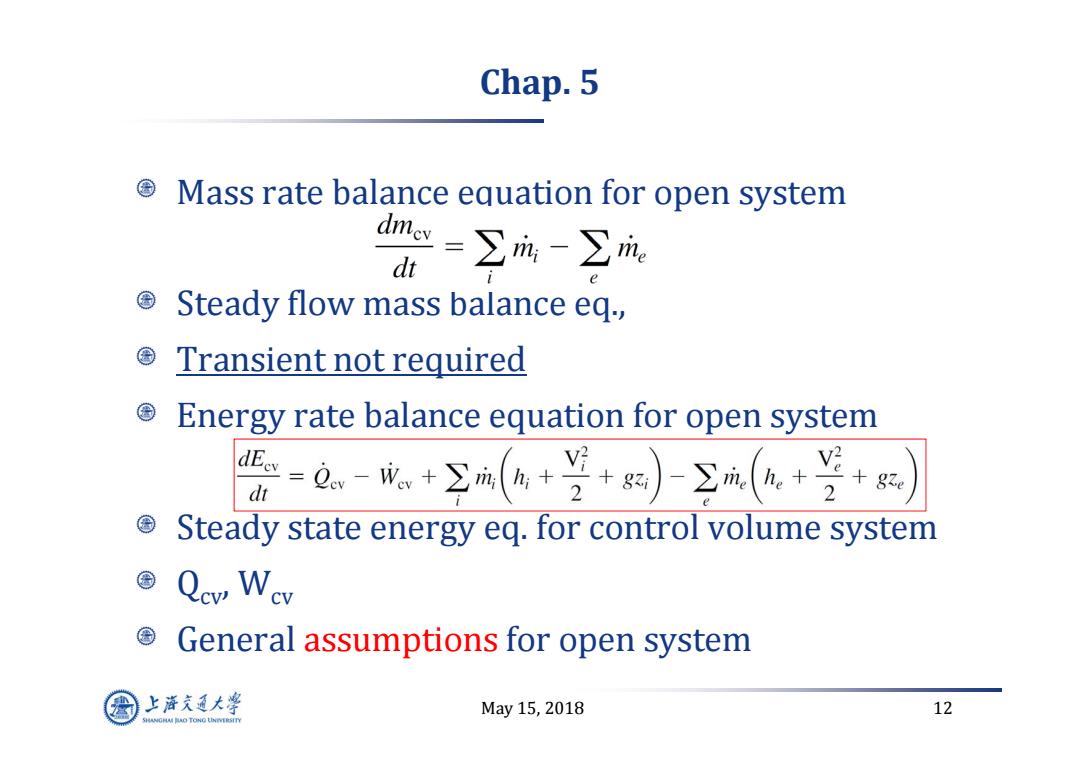
Chap.5 Mass rate balance equation for open system 骨=卫义 Steady flow mass balance eq., Transient not required Energy rate balance equation for open system 医=Q-晚+空(®+学+)-Σ(+兰+ Steady state energy eq.for control volume system Qcv Wev General assumptions for open system 上游充通大率 May15,2018 12 SHANGHAI BAO TONG LINIERSITY
May 15, 2018 12 Chap. 5 Mass rate balance equation for open system Steady flow mass balance eq., Transient not required Energy rate balance equation for open system Steady state energy eq. for control volume system Qcv, Wcv General assumptions for open system