
4 1956 Layered LiMnO2 6
Layered LiMnO2 6

Layered LiMnO2 /986 Synthesis of layered LiMnO,as an electrode for rechargeable lithium batteries A.Robert Armstrong Peter G.Bruce School of Chemistry,University of St Andrews,North Haugh, St Andrews,Fife KY16 9ST,UK Peter G.Bruce Nature volume381,pages499-500(06 June 1996) Here we report the synthesis and electrochemical performance of a new material,layered LiMnO2,which is structurally analogous to LiCoO2.The charge capacity of LiMnO2(~270 mAhg-1)compares well with that of both LiCoO,and LiMn2O4,and preliminary results indicate good stability over repeated charge-discharge cycles. 7
Layered LiMnO2 Nature volume381, pages499–500 (06 June 1996) Here we report the synthesis and electrochemical performance of a new material, layered LiMnO2, which is structurally analogous to LiCoO2. The charge capacity of LiMnO2 (∼270 mAhg–1) compares well with that of both LiCoO2 and LiMn2O4, and preliminary results indicate good stability over repeated charge–discharge cycles. Peter G. Bruce 7
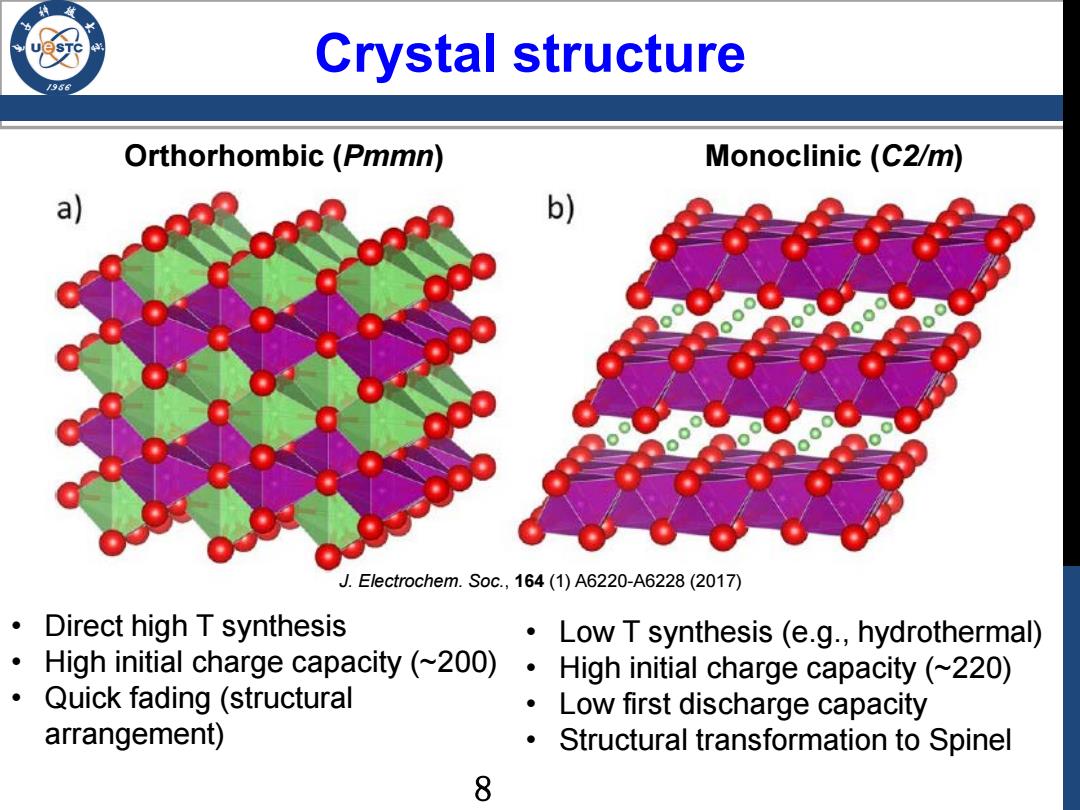
Crystal structure Orthorhombic (Pmmn) Monoclinic (C2/m) a b) J.Electrochem.Soc.,164(1)A6220-A6228(2017) Direct high T synthesis Low T synthesis (e.g.,hydrothermal) High initial charge capacity (~200) High initial charge capacity(~220) Quick fading (structural Low first discharge capacity arrangement) Structural transformation to Spinel 8
Crystal structure Orthorhombic (Pmmn) Monoclinic (C2/m) • Direct high T synthesis • High initial charge capacity (~200) • Quick fading (structural arrangement) • Low T synthesis (e.g., hydrothermal) • High initial charge capacity (~220) • Low first discharge capacity • Structural transformation to Spinel J. Electrochem. Soc., 164 (1) A6220-A6228 (2017) 8