
二、1:n型络合物(简单络合物) 1、逐级稳定常数K稳 金属离子M能与配位剂L逐步形成ML型配合物,每一 步都有配位平衡和相应的稳定常数(逐级稳定常数K稳) [ML] M+L台M K稳1= [M][L] ML+L台M2 K稳2= [ML2] [MLJL] ● [ML,] MLn-1+L台MLn K稳n [ML]L] 山东置子大军 Analytical Chemistry 16
Analytical Chemistry 16 二、 1:n型络合物(简单络合物) 金属离子M能与配位剂L逐步形成MLn型配合物,每一 步都有配位平衡和相应的稳定常数(逐级稳定常数K稳n ) M+ L ML [ ][ ] [ ] 1 M L ML K稳 = ML + L ML2 [ ][ ] [ ] 2 2 ML L ML K稳 = MLn−1 + L MLn [ ][ ] [ ] ML 1 L ML K n n n − 稳 = 1、逐级稳定常数K稳 ● ● ● ● ● ●

2、累积稳定常数Bm 将逐级稳定常数依次相乘,可得到各级累积稳定常数 M+L→M B=K= [ML] M[ M+2L→ML2 B=K德K= [ML2] M[L]2 : ● M+nL→MIn 么-a水-iKa i=1 [M[L” K表示相邻络合物之间的关系 B表示络合物与配体之间的关系 少本理王大家 Analytical Chemistry 17
Analytical Chemistry 17 ● ● ● M+LML M+ 2L ML2 ● ● ● M+nL MLn K 表示相邻络合物之间的关系 表示络合物与配体之间的关系 将逐级稳定常数依次相乘,可得到各级累积稳定常数 2、累积稳定常数 n n 1 1 2 [M][L] [ML ] . . n i n i n = K K K n = K = = 稳 稳 稳 稳 2 2 2 1 2 [M][L] [ML ] = K稳 .K稳 = [M][L] [ML] 1 = K稳1 =
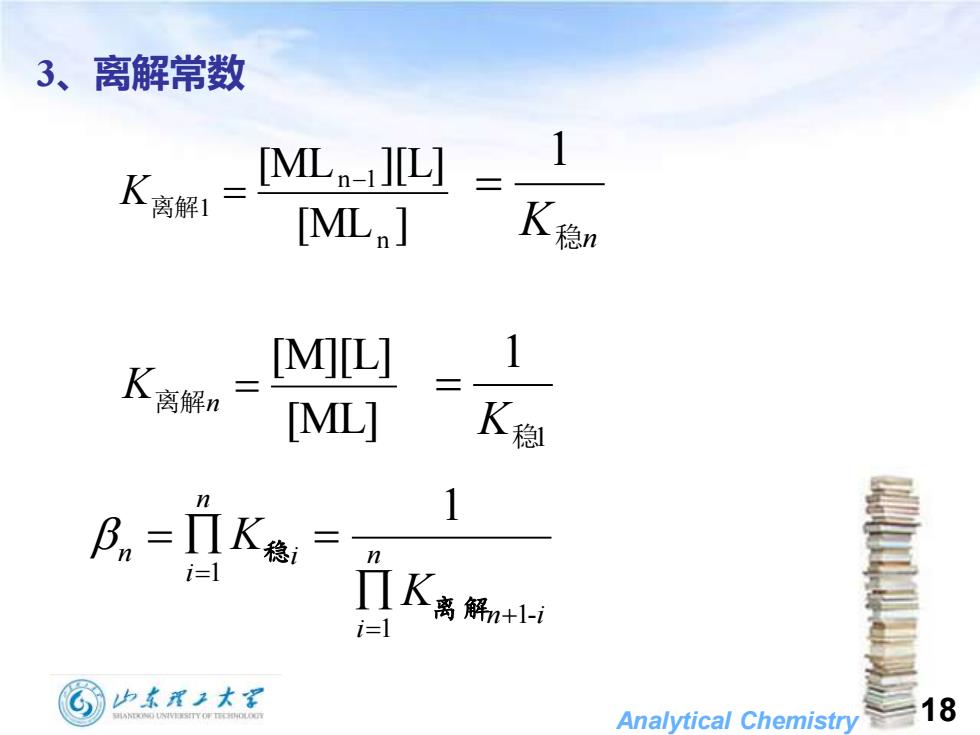
3、离解常数 [ML]L] =_ [ML 飞稳n K离解n [M][L] 1 [ML] 飞稳 Bn=1K德,= 1 i-1 门K禹解+1i i=1 少东理子大军 Analytical Chemistry18
Analytical Chemistry 18 3、离解常数 [ML ] [ML ][L] n n 1 1 − K离解 = K稳n 1 = [ML] [M][L] = K离解n 1 1 K稳 = n i n i i n i n K K 1- 1 1 1 + = = = = 离 解 稳

622 溶液中各级络合物的分布分数 多元络合物 M+L台MI [ML]=B[M][L] M+2L→ML2 [ML2]=B2 [M][L]2 M+nL→MLn [MLJ=BM][LI" 物料平衡 CM [M]+[ML]+[ML2]+.+[ML CM =[M]+B[M][L]+B[M][L]+.+B,[M][L]" cM=[M1+∑B,[LD δ,=f(B,IL]) 分布分数 =IM 比较酸的分布分数: β,[L,] CM 少东理) 8=f(Ka:PH) 1+∑B,L]) = 19
Analytical Chemistry 19 多元络合物 分布分数 (1 [L ] ) [L ] [M](1 [L ] ) [ML ] [M][L ] 1 1 M = = + = + = = n i i i i i n i i i i i i i i i i i c 物料平衡 [M] [ML] [ML ] . [ML ] M 2 n c = + + + + 2 M 1 2 [M] [M] [M] [L] [L] . [M][L]n n c = + + + + M 1 [M] 1 [L] n i i i c = = + ( ) 溶液中各级络合物的分布分数 [ML] = 1 [M] [L] [ML2 ]= 2 [M] [L]2 [MLn ]= n [M] [L]n M+LML M+ 2L ML2 M+nL MLn f ( ,[L]) i = i f (K , pH) i = a 比较酸的分布分数:
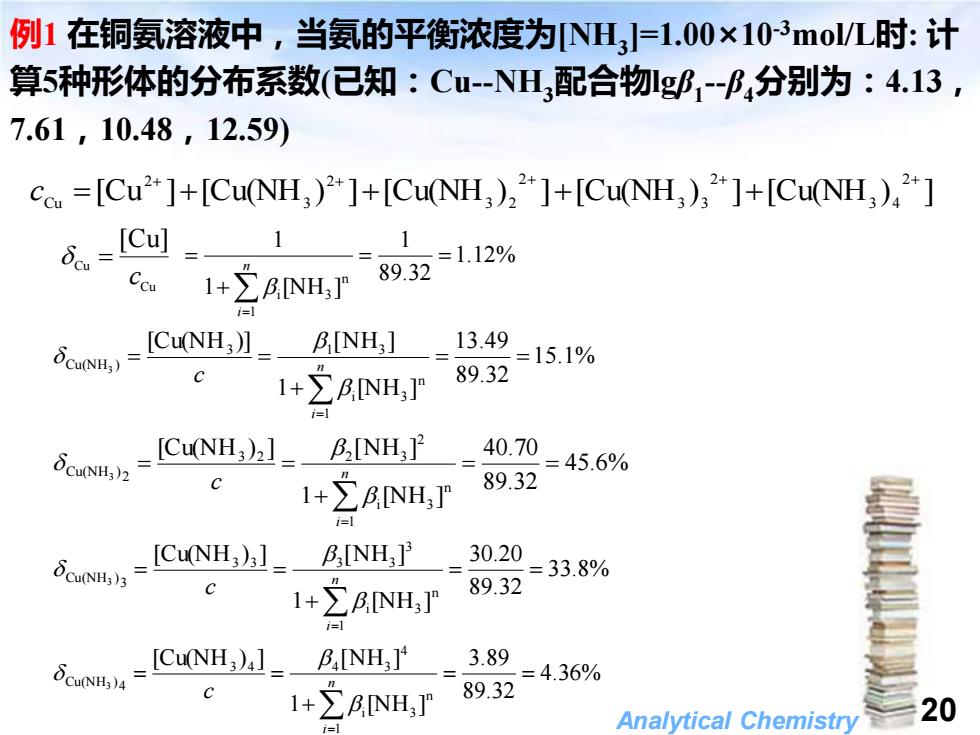
例1在铜氨溶液中,当氨的平衡浓度为NH3=1.00×103mol/L时:计 算5种形体的分布系数(已知:C-NH3配合物g1-B,分别为:4.13, 7.61,10.48,12.59) c.=[Cu2+]+[CuNH)2]+[CuNH)2*]+[CuNH3),2*]+[CuNH,)42+] o=ICu] 1 1 89.32 =1.12% c1+∑B,NH] =1 N-ICNH,】。- BNH,】=13.49 89.32 =15.1% 1I+∑ANH,]P i=l δcuNH,2 _ICu(NH,bl_B,NH,_=4070=45.6% 1+NHT 89.32 i=1 Cu(NH3)3 =[CuNH,]-B,NH,P =30.20=33.8% 1+NHT 89.32 1=】 δcuNH4 -[Cu(NH,)]_B,INH,] 3.89 =4.36% 1+∑BNH,F 89.32 Analytical Chemistry 20 i=1
Analytical Chemistry 20 4.36% 89.32 3.89 1 [NH ] [Cu(NH ) ] [NH ] 1 n i 3 4 3 4 4 3 4 Cu(NH ) 3 = = + = = = n i c 例1 在铜氨溶液中,当氨的平衡浓度为[NH3 ]=1.00×10-3mol/L时: 计 算5种形体的分布系数(已知:Cu-NH3配合物lgβ1 -β4分别为:4.13, 7.61,10.48,12.59) [Cu ] [Cu(NH ) ] [Cu(NH ) ] [Cu(NH ) ] [Cu(NH ) ] 2 3 4 2 3 3 2 3 2 2 3 2 Cu + + + + + c = + + + + Cu Cu [Cu] c = 1.12% 89.32 1 1 [NH ] 1 1 n i 3 = = + = = n i 15.1% 89.32 13.49 1 [NH ] [Cu(NH )] [N H ] 1 n i 3 3 1 3 Cu(NH ) 3 = = + = = = n i c 45.6% 89.32 40.70 1 [NH ] [Cu(NH ) ] [NH ] 1 n i 3 2 3 2 2 3 2 Cu(NH ) 3 = = + = = = n i c 33.8% 89.32 30.20 1 [NH ] [Cu(NH ) ] [NH ] 1 n i 3 3 3 3 3 3 3 Cu(NH ) 3 = = + = = = n i c