
Table 2.4.Typical HH coupling constants(Hz)of some units in alicycles,alkenes and alkynes2 3JHH JHH JHH geminal protons protons with w-relationships H -12.5 -6.0 H H-2.5 4.5 5.5 -2.0 2.5 16.5 =-H-30 三一H 1.0 H
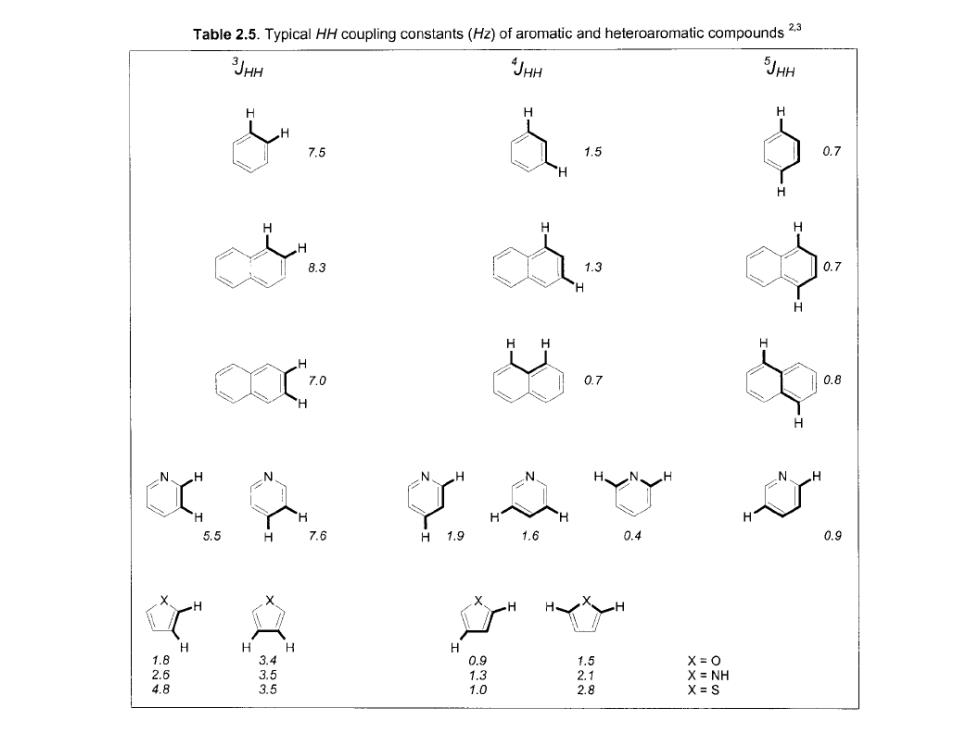
Table 2.5.Typical HH coupling constants(Hz)of aromatic and heteroaromatic compounds23 3JHH JHH 5JHH 7 0.7 0.9 H 1.8 3.4 0.9 X=0

Hz Hz g 18 16 14 14 12 12 10 10 8 8 6 6 4 4 2 2 0 0 0 306090120150 180° p Karplus-Conroy Eq.3Jhh=acos2Φ-0.28(a=10ifΦ<90;a=15if4>90) Martin Karplus:2013 Nobel laureate.(CHARMM,量子力学和经典力学结合计算生 物学问题)
Karplus-Conroy Eq. 3JHH = a cos2f - 0.28 (a =10 if f<90; a=15 if f >90) Martin Karplus: 2013 Nobel laureate. (CHARMM, 量子力学和经典力学结合计算生 物学问题)
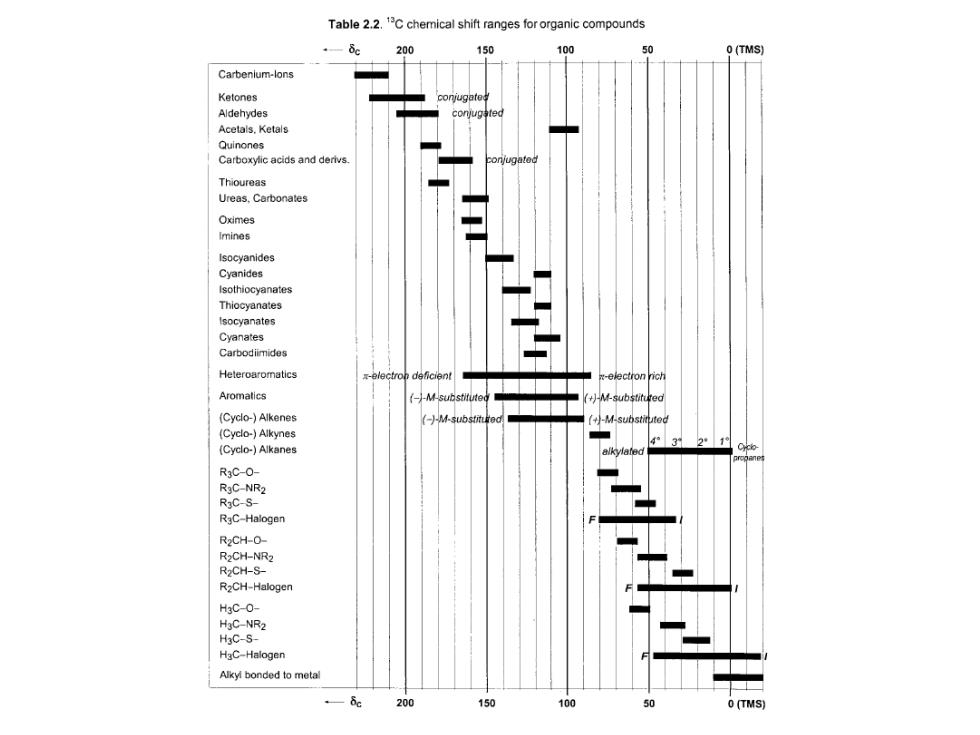
Table 2.2.1C chemical shift ranges for organic compounds 200 150 O(TMS) Carbenlum-lons Ketones Aldehydes Acetals.Ketals Quinones Carboxylic acids and derivs. Thioureas Ureas.Carbonates Oximes Imines Cyanides Thiocyanates tsocyanates Cyanates Carbodimides Heteroaromatics Aromatics (Cyclo-)Alkenes -1-M-sub (Cyclo-)Alkynes (Cyclo-)Alkanes R3C-0- R3C-NR2 RaC-S- R3C-Halogen R2CH-0- R2CH-NR2 R2CH-S- R2CH-Haloger H3C-0 HgC-NR2 H3C-S- H3C-Halogen Alkyi bonded to meta 200 150 100 O (TMS
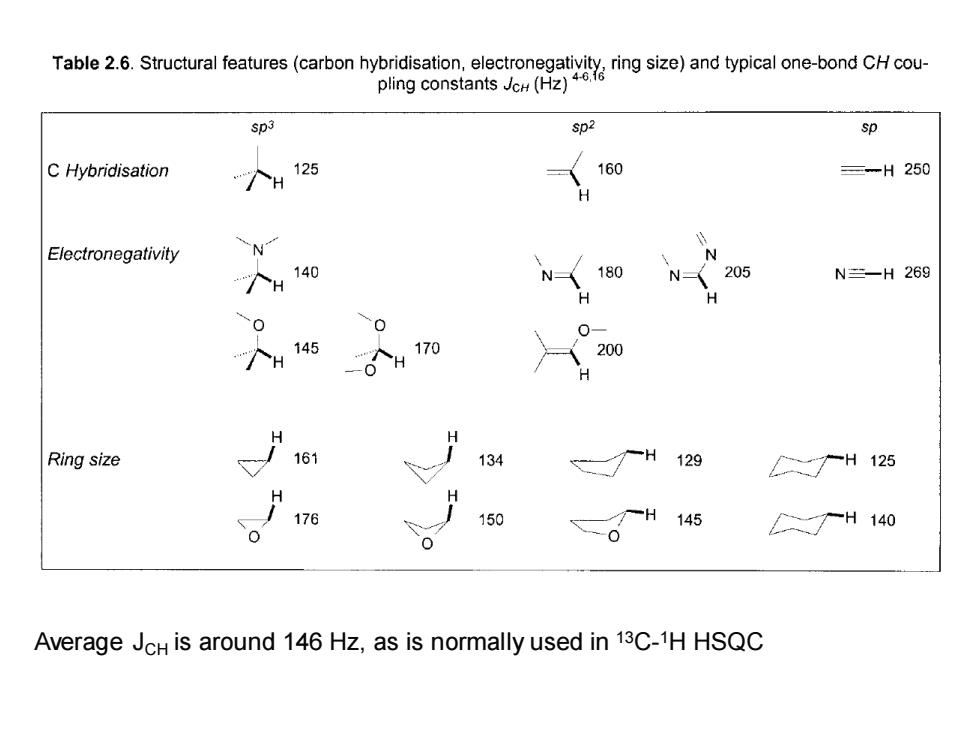
Tabl2.Structurau (hyiin)ad typical o-bond Sp3 sp2 sp C Hybridisation 125 160 三-H250 H Electronegativity N N 个H 140 N 180 N人 205 N=H269 0 0 "个H145 O个H 170 200 Ring size 161 134 129 个H125 176 150 145 H140 Average JcH is around 146 Hz,as is normally used in 13C-1H HSQC
Average JCH is around 146 Hz, as is normally used in 13C- 1H HSQC