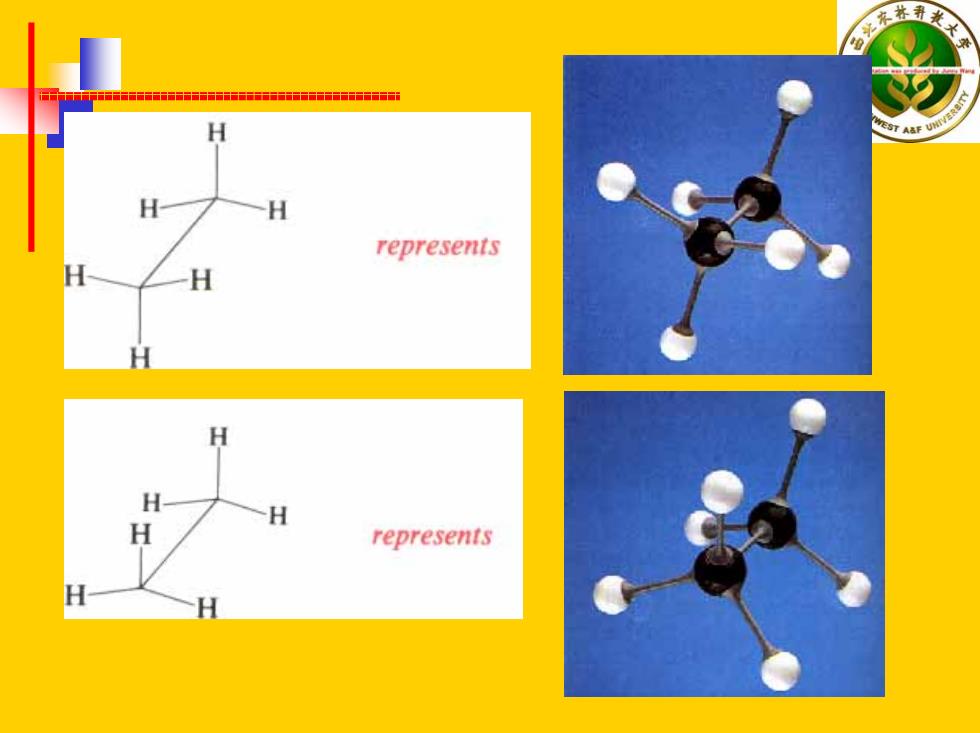
H H represents H H H represents

H H represents H H represents H H H H 必乙烷构象转化中的能量变化 公丁烷转化中的能量变化 必扭转张力
乙烷构象转化中的能量变化 丁烷转化中的能量变化 扭转张力
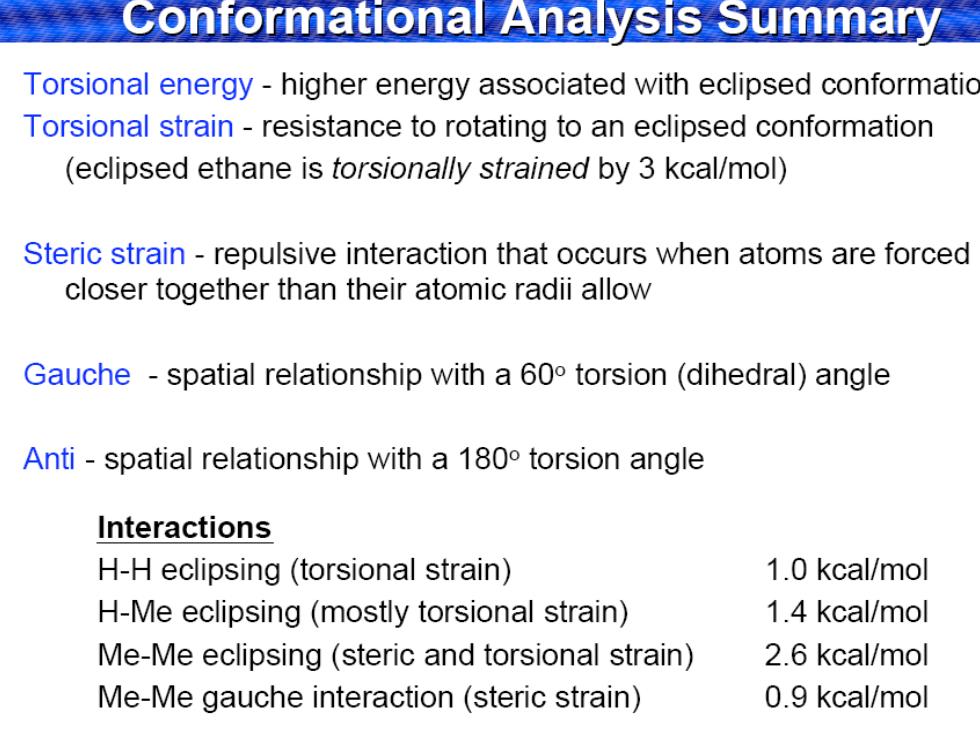
Conformational Analysis Summary Torsional energy-higher energy associated with eclipsed conformatio Torsional strain-resistance to rotating to an eclipsed conformation (eclipsed ethane is torsionally strained by 3 kcal/mol) Steric strain-repulsive interaction that occurs when atoms are forced closer together than their atomic radii allow Gauche -spatial relationship with a 60 torsion(dihedral)angle Anti -spatial relationship with a 1800 torsion angle Interactions H-H eclipsing (torsional strain) 1.0 kcal/mol H-Me eclipsing(mostly torsional strain) 1.4 kcal/mol Me-Me eclipsing(steric and torsional strain) 2.6 kcal/mol Me-Me gauche interaction(steric strain) 0.9 kcal/mol

D2-Confermation Analysis of Cycloalkanes---Cyclohexane Preferential Conformation(优势构象) Cyclohexane Substituted Cyclohexanes
D2 Conformation Analysis of Cycloalkanes---Cyclohexane Preferential Conformation (优势构象 ) Cyclohexane Substituted Cyclohexanes

Flat Cyclohexane? Several bad H-H eclipsing interactions! Cyclohexane is not planar!
Flat Cyclohexane? Several bad H-H eclipsing interactions! Cyclohexane is not planar!