
Atomic Structure:Orbitals How are the electrons distributed in an atom? ■ Quantum mechanics:behavior of a specific electron in an atoms is descrbied by a mathematical expression called a wave equation The solution of the wave equation is called an orbital An orbital describes the volume of space around a nucleus where an electron is mostly likely to be found
Atomic Structure: Orbitals Quantum mechanics: behavior of a specific electron in an atoms is descrbied by a mathematical expression called a wave equation The solution of the wave equation is called an orbital An orbital describes the volume of space around a nucleus where an electron is mostly likely to be found How are the electrons distributed in an atom?

Shapes of Atomic Orbitals Four different kinds of orbitals ■Denoted s,p,d,andf s and p orbitals most important in organic chemistry s orbitals:spherical,nucleus at center p orbitals:dumbbell-shaped,nucleus at middle An s orbital A p orbital A d orbital
Shapes of Atomic Orbitals Four different kinds of orbitals Denoted s, p, d, and f s and p orbitals most important in organic chemistry s orbitals: spherical, nucleus at center p orbitals: dumbbell-shaped, nucleus at middle

p-Orbitals ■The three perpendicular p orbitals,px,py,and pz,are of equal energy A 2px orbital A 2py orbital ■Lobes of a p orbital are separated by region of zero electron density,a node Thomson-Brooks Col A 2pz orbital Three 2p orbitals
p-Orbitals The three perpendicular p orbitals, p x, p y, and p z, are of equal energy Lobes of a p orbital are separated by region of zero electron density, a node

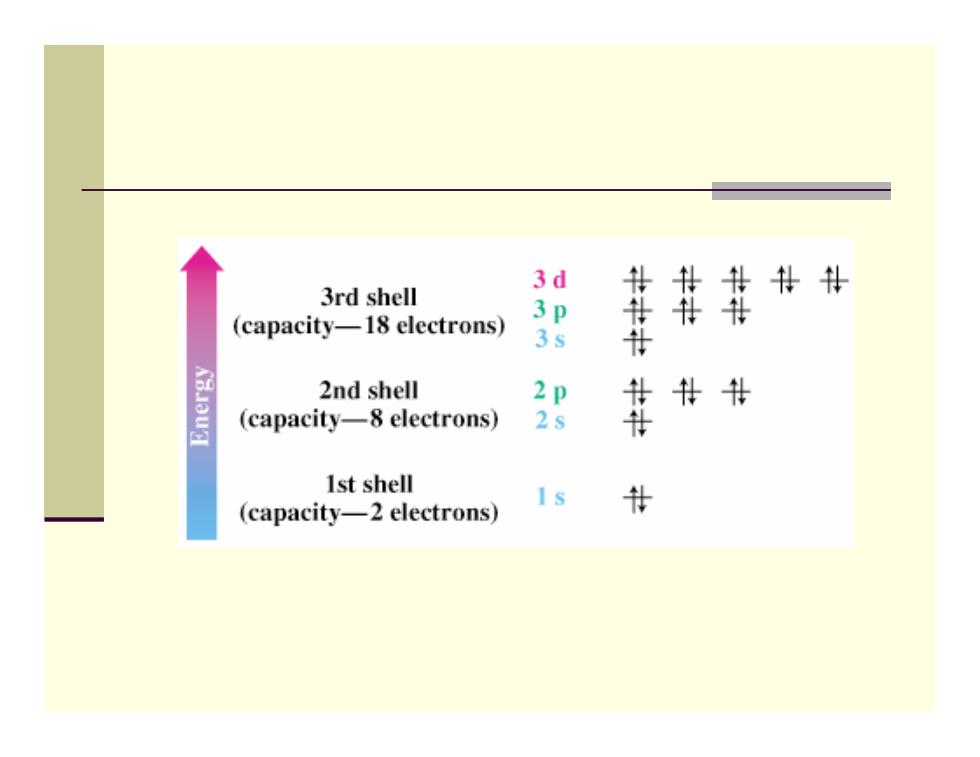
Orbitals and Shells ■ Orbitals are organized into shells of increasing size and energy ■ Different shells contain different numbers and kinds of orbitals Each orbital can be occupied by two electrons First shell contains one s orbital,denoted 1s ■ Second shell contains one s orbital(2s)and three p orbitals(2p) Third shell contains an s orbital(3s),three p orbitals (3p),and five d orbitals (3d)
Orbitals and Shells Orbitals are organized into shells of increasing size and energy Different shells contain different numbers and kinds of orbitals Each orbital can be occupied by two electrons First shell contains one s orbital, denoted 1 s Second shell contains one s orbital ( 2 s) and three p orbitals ( 2 p ) Third shell contains an s orbital ( 3 s), three p orbitals ( 3 p), and five d orbitals (3 d )
3d 3rd shell # (capacity-18 electrons) 3s 2nd shell 2 (capacity-8 electrons) 2s 鞋 1st shell (capacity-2 electrons) #